Controlled elevated temperatures during early-mid gestation cause placental insufficiency and implications for fetal growth in pregnant pigs
- PMID: 33244103
- PMCID: PMC7691357
- DOI: 10.1038/s41598-020-77647-1
Controlled elevated temperatures during early-mid gestation cause placental insufficiency and implications for fetal growth in pregnant pigs
Abstract
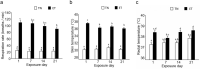
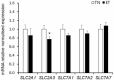
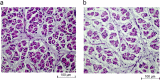
It is known that pig offspring born from pregnant pigs exposed to elevated ambient temperatures during gestation have altered phenotypes, possibly due to placental insufficiency and impaired fetal growth. Therefore, the objective of this study was to quantify the effect of maternal heat exposure during early-mid gestation, when pig placentae grow heavily, on placental and fetal development. Fifteen pregnant pigs were allocated to thermoneutral (TN; 20 °C; n = 7) or cyclic elevated temperature conditions (ET; 28 to 33 °C; n = 8) from d40 to d60 of gestation. Following euthanasia of the pigs on d60, placental and fetal morphometry and biochemistry were measured. Compared to TN fetuses, ET fetuses had increased (P = 0.041) placental weights and a lower (P = 0.013) placental efficiency (fetal/placental weight), although fetal weights were not significantly different. Fetuses from ET pigs had reduced (P = 0.032) M. longissimus fibre number density and a thicker (P = 0.017) placental epithelial layer compared to their TN counterparts. Elevated temperatures decreased (P = 0.026) placental mRNA expression of a glucose transporter (GLUT-3) and increased (P = 0.037) placental IGF-2 mRNA expression. In conclusion, controlled elevated temperatures between d40 to d60 of gestation reduced pig placental efficiency, resulting in compensatory growth of the placentae to maintain fetal development. Placental insufficiency during early-mid gestation may have implications for fetal development, possibly causing a long-term phenotypic change of the progeny.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Maternal Heat Stress Alters Expression of Genes Associated with Nutrient Transport Activity and Metabolism in Female Placentae from Mid-Gestating Pigs.Int J Mol Sci. 2021 Apr 16;22(8):4147. doi: 10.3390/ijms22084147. Int J Mol Sci. 2021. PMID: 33923747 Free PMC article.
-
Altered arterial concentrations of placental hormones during maximal placental growth in a model of placental insufficiency.J Endocrinol. 1999 Sep;162(3):433-42. doi: 10.1677/joe.0.1620433. J Endocrinol. 1999. PMID: 10467235
-
Altered placental and fetal expression of IGFs and IGF-binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid-pregnancy.Pediatr Res. 2006 Nov;60(5):507-12. doi: 10.1203/01.PDR.0000242364.78002.71. Epub 2006 Sep 11. Pediatr Res. 2006. PMID: 16966353
-
Distinct actions of insulin-like growth factors (IGFs) on placental development and fetal growth: lessons from mice and guinea pigs.Placenta. 2008 Mar;29 Suppl A:S42-7. doi: 10.1016/j.placenta.2007.12.002. Epub 2008 Jan 11. Placenta. 2008. PMID: 18191196 Review.
-
Fetal undernutrition, placental insufficiency, and pancreatic β-cell development programming in utero.Am J Physiol Regul Integr Comp Physiol. 2018 Nov 1;315(5):R867-R878. doi: 10.1152/ajpregu.00072.2018. Epub 2018 Aug 15. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 30110175 Free PMC article. Review.
Cited by
-
Effect of dietary resveratrol on placental function and reproductive performance of late pregnancy sows.Front Nutr. 2022 Oct 27;9:1001031. doi: 10.3389/fnut.2022.1001031. eCollection 2022. Front Nutr. 2022. PMID: 36407549 Free PMC article.
-
Effect of Dietary Supplementation with Mannose Oligosaccharides on the Body Condition, Lactation Performance and Their Offspring of Heat-Stressed Sows.Animals (Basel). 2022 May 29;12(11):1397. doi: 10.3390/ani12111397. Animals (Basel). 2022. PMID: 35681861 Free PMC article.
-
Gestational heat stress alters skeletal muscle gene expression profiles and vascularity in fetal pigs in a sexually dimorphic manner.J Anim Sci Biotechnol. 2022 Jul 15;13(1):76. doi: 10.1186/s40104-022-00730-2. J Anim Sci Biotechnol. 2022. PMID: 35836286 Free PMC article.
-
Late gestation fetal hypothyroidism alters cell cycle regulation across multiple organ systems.BMC Vet Res. 2024 Jun 20;20(1):268. doi: 10.1186/s12917-024-04102-y. BMC Vet Res. 2024. PMID: 38902754 Free PMC article.
-
Welfare of pigs during transport.EFSA J. 2022 Sep 7;20(9):e07445. doi: 10.2903/j.efsa.2022.7445. eCollection 2022 Sep. EFSA J. 2022. PMID: 36092763 Free PMC article.
References
-
- King AD, Karoly DJ, Henley BJ. Australian climate extremes at 1.5 °C and 2 °C of global warming. Nat. Clim. Change. 2017;7:412–416. doi: 10.1038/nclimate3296. - DOI
-
- Henry B, Charmley E, Eckard R, Gaughan JB, Hegarty R. Livestock production in a changing climate: adaptation and mitigation research in Australia. Crop. Pasture. Sci. 2012;63:191–202. doi: 10.1071/CP11169. - DOI
-
- Prunier A, Quesnel H, de Braganca MM, Kermabon AY. Environmental and seasonal influences on the return-to-oestrus after weaning in primiparous sows: a review. Livest. Prod. Sci. 1996;45:103–110. doi: 10.1016/0301-6226(96)00007-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

