Redesigned reporter gene for improved proton exchange-based molecular MRI contrast
- PMID: 33244130
- PMCID: PMC7692519
- DOI: 10.1038/s41598-020-77576-z
Redesigned reporter gene for improved proton exchange-based molecular MRI contrast
Abstract
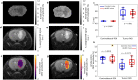
Reporter gene imaging allows for non-invasive monitoring of molecular processes in living cells, providing insights on the mechanisms underlying pathology and therapy. A lysine-rich protein (LRP) chemical exchange saturation transfer (CEST) MRI reporter gene has previously been developed and used to image tumor cells, cardiac viral gene transfer, and oncolytic virotherapy. However, the highly repetitive nature of the LRP reporter gene sequence leads to DNA recombination events and the expression of a range of truncated LRP protein fragments, thereby greatly limiting the CEST sensitivity. Here we report the use of a redesigned LRP reporter (rdLRP), aimed to provide excellent stability and CEST sensitivity. The rdLRP contains no DNA repeats or GC rich regions and 30% less positively charged amino-acids. RT-PCR of cell lysates transfected with rdLRP demonstrated a stable reporter gene with a single distinct band corresponding to full-length DNA. A distinct increase in CEST-MRI contrast was obtained in cell lysates of rdLRP transfected cells and in in vivo LRP expressing mouse brain tumors ([Formula: see text], n = 10).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Campan M., et al. Ferritin as a reporter gene for in vivo tracking of stem cells by 1.5-T cardiac MRI in a rat model of myocardial infarction. Am. J. Physiol. 2011;300:H2238–H2250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01NS098231/NH/NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- R01 NS098231/NS/NINDS NIH HHS/United States
- R01 NS104306/NS/NINDS NIH HHS/United States
- S10 OD010705/OD/NIH HHS/United States
- G20 RR031051/RR/NCRR NIH HHS/United States
- R01 CA203873/CA/NCI NIH HHS/United States
- P01 CA163205/CA/NCI NIH HHS/United States
- 836752/MCCC_/Marie Curie/United Kingdom
- R01 NS110942/NS/NINDS NIH HHS/United States
- R01 EB031008/EB/NIBIB NIH HHS/United States
- R01-CA203873/NH/NIH HHS/United States
- P41 EB024495/EB/NIBIB NIH HHS/United States
- 1R01NS110942-01A1/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

