Advances in transcriptome analysis of human brain aging
- PMID: 33244150
- PMCID: PMC8080664
- DOI: 10.1038/s12276-020-00522-6
Advances in transcriptome analysis of human brain aging
Abstract
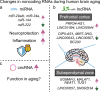
Aging is associated with gradual deterioration of physiological and biochemical functions, including cognitive decline. Transcriptome profiling of brain samples from individuals of varying ages has identified the whole-transcriptome changes that underlie age-associated cognitive declines. In this review, we discuss transcriptome-based research on human brain aging performed by using microarray and RNA sequencing analyses. Overall, decreased synaptic function and increased immune function are prevalent in most regions of the aged brain. Age-associated gene expression changes are also cell dependent and region dependent and are affected by genotype. In addition, the transcriptome changes that occur during brain aging include different splicing events, intersample heterogeneity, and altered levels of various types of noncoding RNAs. Establishing transcriptome-based hallmarks of human brain aging will improve the understanding of cognitive aging and neurodegenerative diseases and eventually lead to interventions that delay or prevent brain aging.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

