The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection
- PMID: 33244161
- PMCID: PMC7690066
- DOI: 10.1038/s41385-020-00359-2
The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection
Abstract
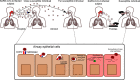
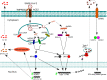
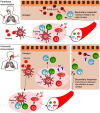
The novel coronavirus SARS-CoV-2 enters into the human body mainly through the ACE2 + TMPRSS2+ nasal epithelial cells. The initial host response to this pathogen occurs in a peculiar immune microenvironment that, starting from the Nasopharynx-Associated Lymphoid Tissue (NALT) system, is the product of a long evolutionary process that is aimed to first recognize exogenous airborne agents. In the present work, we want to critically review the latest molecular and cellular findings on the mucosal response to SARS-CoV-2 in the nasal cavity and in NALT, and to analyze its impact in the subsequent course of COVID-19. Finally, we want to explore the possibility that the regulation of the systemic inflammatory network against the virus can be modulated starting from the initial phases of the nasal and nasopharyngeal response and this may have several clinical and epidemiological implications starting from a mucosal vaccine development.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal. Immunol. 2008;1:31–37. 1:CAS:528:DC%2BD1cXhtlWhuw%3D%3D, 19079158. - PubMed
-
- Elad D, Wolf M, Keck T. Air-conditioning in the human nasal cavity. Respir. Physiol. Neurobiol. 2008;163:121–127. 18565805. - PubMed
-
- Newsome H, et al. Clinical importance of nasal air conditioning: a review of the literature. Am. J. Rhinol. Allergy. 2019;33:763–769. 31291132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

