Rejuveinix Shows a Favorable Clinical Safety Profile in Human Subjects and Exhibits Potent Preclinical Protective Activity in the Lipopolysaccharide-Galactosamine Mouse Model of Acute Respiratory Distress Syndrome and Multi-Organ Failure
- PMID: 33244300
- PMCID: PMC7683794
- DOI: 10.3389/fphar.2020.594321
Rejuveinix Shows a Favorable Clinical Safety Profile in Human Subjects and Exhibits Potent Preclinical Protective Activity in the Lipopolysaccharide-Galactosamine Mouse Model of Acute Respiratory Distress Syndrome and Multi-Organ Failure
Abstract
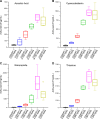
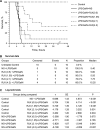
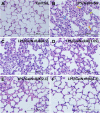
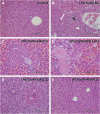
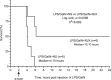
Background: New treatment platforms that can prevent acute respiratory distress syndrome (ARDS) or reduce its mortality rate in high-risk coronavirus disease 2019 (COVID-19) patients, such as those with an underlying cancer, are urgently needed. Rejuveinix (RJX) is an intravenous formulation of anti-oxidants and anti-inflammatory agents. Its active ingredients include ascorbic acid, cyanocobalamin, thiamine hydrochloride, riboflavin 5' phosphate, niacinamide, pyridoxine hydrochloride, and calcium D-pantothenate. RJX is being developed as an anti-inflammatory and anti-oxidant treatment platform for patients with sepsis, including COVID-19 patients with viral sepsis and ARDS. Here, we report its clinical safety profile in a phase 1 clinical study (ClinicalTrials.gov Identifier: NCT03680105) and its potent protective activity in the lipopolysaccharide galactosamine (LPS-GalN) mouse model of ARDS. Methods: A phase 1, double-blind, placebo-controlled, randomized, two-part, ascending dose-escalation study was performed in participating 76 healthy volunteer human subjects in compliance with the ICH (E6) good clinical practice guidelines to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of RJX (Protocol No. RPI003; ClinicalTrials.gov Identifier: NCT03680105). The ability of RJX to prevent fatal shock, ARDS, and multi-organ failure was examined in the well-established LPS-GalN mouse model of sepsis and ARDS. Standard methods were employed for the statistical analysis of data in both studies. Findings: In the phase 1 clinical study, no participant developed serious adverse events (SAEs) or Grade 3-Grade 4 adverse events (AEs) or prematurely discontinued participation in the study. In the non-clinical study, RJX exhibited potent and dose-dependent protective activity, decreased the inflammatory cytokine responses (interleukin-6, tumor necrosis factor alpha, transforming growth factor beta), and improved survival in the LPS-GalN mouse model of sepsis and ARDS. Histopathological examinations showed that RJX attenuated the LPS-GalN induced acute lung injury (ALI) and pulmonary edema as well as liver damage. Conclusion: RJX showed a very favorable safety profile and tolerability in human subjects. It shows potential to favorably affect the clinical course of high-risk COVID-19 by preventing ARDS and its complications.
Keywords: COVID-19; acute lung injury; cancer; cytokine release syndrome; multi-organ dysfunction; pneumonia; sepsis.
Copyright © 2020 Uckun, Carlson, Orhan, Powell, Pizzimenti, van Wyk, Ozercan, Volk and Sahin.
Figures



References
-
- Bi Q., Hong C., Meng J., Wu Z., Zhou P., Ye C., et al. (2020). Characterizing clinical progression of COVID-19 among patients in Shenzhen, China: an observational cohort study. medRxiv 10.1101/2020.04.22.20076190 - DOI
-
- Cabala K., Earabino J., Pacult P., Uckun F. M. (2020). Rationale for A randomized, placebo-controlled, phase 2 study of Rejuveinix (RJX) in COVID-19 patients with acute lung injury and hypoxemic respiratory failure. Clin. Invest. 10 (2), 185–189.
-
- Carver C., Jones N. (2020). Are there risk factors and preventive interventions for acute respiratory distress syndrome (ARDS) in COVID-19. COVID-19 Evidence Service, Centre for Evidence-Based Medicine (CEBM) 2020 Jun 8; Edinburg. Available at: https://www.cebm.net/covid-19/are-there-risk-factors-and-preventative-in....
Associated data
LinkOut - more resources
Full Text Sources
Medical

