A REDCap-based model for electronic consent (eConsent): Moving toward a more personalized consent
- PMID: 33244416
- PMCID: PMC7681162
- DOI: 10.1017/cts.2020.30
A REDCap-based model for electronic consent (eConsent): Moving toward a more personalized consent
Abstract
Introduction: The updated common rule, for human subjects research, requires that consents "begin with a 'concise and focused' presentation of the key information that will most likely help someone make a decision about whether to participate in a study" (Menikoff, Kaneshiro, Pritchard. The New England Journal of Medicine. 2017; 376(7): 613-615.). We utilized a community-engaged technology development approach to inform feature options within the REDCap software platform centered around collection and storage of electronic consent (eConsent) to address issues of transparency, clinical trial efficiency, and regulatory compliance for informed consent (Harris, et al. Journal of Biomedical Informatics 2009; 42(2): 377-381.). eConsent may also improve recruitment and retention in clinical research studies by addressing: (1) barriers for accessing rural populations by facilitating remote consent and (2) cultural and literacy barriers by including optional explanatory material (e.g., defining terms by hovering over them with the cursor) or the choice of displaying different videos/images based on participant's race, ethnicity, or educational level (Phillippi, et al. Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2018; 47(4): 529-534.).
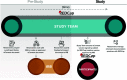
Methods: We developed and pilot tested our eConsent framework to provide a personalized consent experience whereby users are guided through a consent document that utilizes avatars, contextual glossary information supplements, and videos, to facilitate communication of information.
Results: The eConsent framework includes a portfolio of eight features, reviewed by community stakeholders, and tested at two academic medical centers.
Conclusions: Early adoption and utilization of this eConsent framework have demonstrated acceptability. Next steps will emphasize testing efficacy of features to improve participant engagement with the consent process.
Keywords: Consent; REDCap; community engagement; electronic; personalized.
© The Association for Clinical and Translational Science 2020.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Use of Electronic Informed Consent in Clinical Investigations Questions and Answers Guidance for Industry Draft Guidance [Internet]. United States Food and Drug Administration (FDA), 2015. (https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat...)
-
- Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. Journal of the American Medical Association. 2004; 292(13): 1593–1601. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
