A Case-Control Study of the 2019 Influenza Vaccine and Incidence of COVID-19 Among Healthcare Workers
- PMID: 33244671
- PMCID: PMC7690338
- DOI: 10.1007/s10875-020-00925-0
A Case-Control Study of the 2019 Influenza Vaccine and Incidence of COVID-19 Among Healthcare Workers
Abstract
Purpose: The influenza vaccine is essential in reducing the influenza burden, especially among healthcare workers (HCW). Experimental studies suggest both coronaviruses and influenza viruses engage with the angiotensin-converting enzyme 2 (ACE 2) and tetraspanin antibodies, and that ACE 2 tetraspanin antibodies in turn may inhibit both coronavirus and low-pathogenicity influenza A viruses (LP IAV) infections. This study aims to investigate the potential clinical association between receiving the 2019 influenza vaccine and the incidence of COVID-19 among HCW.
Methods: We designed a case-control study within a hospital setting in Iran when it became a center for treating COVID-19 patients. We collected data and calculated relevant incidence and associative measures among HCW who had received the 2019 influenza vaccine as compared to HCW who had not received the vaccine.
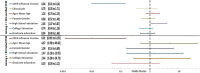
Results: Our total sample size was 261 HCW. Of 80 COVID-19 incident cases, three cases had received the influenza vaccine, while 87 of 181 controls had received the vaccine. The odds ratio (OR) and confidence interval (CI) of being vaccinated were 0.04 (95% CI: 0.01 to 0.14) among COVID-19 cases as compared to controls.
Conclusions: Significant findings suggest that the 2019 influenza vaccine may have a protective association against COVID-19 among HCW.
Keywords: COVID-19; case–control; coronavirus; healthcare workers; influenza; vaccine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, Stensballe L, Diness BR, Lausch KR, Lund N, Biering-Sørensen S, Whittle H, Benn CS. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204(2):245–252. doi: 10.1093/infdis/jir240. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

