Pandemics: past, present, future: That is like choosing between cholera and plague
- PMID: 33244837
- PMCID: PMC7753327
- DOI: 10.1111/apm.13098
Pandemics: past, present, future: That is like choosing between cholera and plague
Abstract
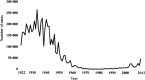
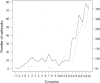
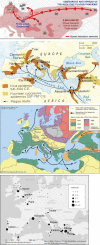
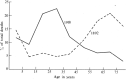
The major epidemic and pandemic diseases that have bothered humans since the Neolithic Age and Bronze Age are surveyed. Many of these pandemics are zoonotic infections, and the mathematical modeling of such infections is illustrated. Plague, cholera, syphilis, influenza, SARS, MERS, COVID-19, and new potential epidemic and pandemic infections and their consequences are described and the background for the spread of acute and chronic infections and the transition to endemic infections is discussed. The way we can prevent and fight pandemics is illustrated from the old and new well-known pandemics. Surprisingly, the political reactions through different periods have not changed much during the centuries.
Keywords: COVID-19; MERS; Pandemics; SARS; cholera; epidemics; influenza; plague.
© 2020 The Authors. APMIS published by John Wiley & Sons Ltd on behalf of Scandinavian Societies for Medical Microbiology and Pathology.
Figures





References
-
- Morabia A. Epidemic and population pattern in the Chinese Empire (243 B.C.E. to 1911 C.E.): quantitative analysis of a unique but neglected epidemic catalogue. Epidemiol Infect 2009;137:1361–8. - PubMed
-
- Gjedde A. Peter Ludvig Panums videnskabelige indsats. Copenhagen, Denmark: Costers Bogtrykkeri, 1971: 256.
-
- Muscat M, Bang H, Wohlfahrt J, Gliesmann S, Mølbak K. Measles in Europe: an epidemiological assessment. Lancet 2009;373:383–9. - PubMed
-
- Clark T. Changing pertussis epidemiology: everything old is new again. J Infect Dis 2014;209:978–81. - PubMed
-
- Nørskov‐Lauritsen N. Et paradigmeskifte. Bibl Læg 2016;208:304–23.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

