In-bore MRI targeted biopsy
- PMID: 33245071
- PMCID: PMC8023080
- DOI: 10.23750/abm.v91i10-S.10251
In-bore MRI targeted biopsy
Abstract
Clinical suspicion of Prostate Cancer (PCa) is largely based on increased prostate specific antigen (PSA) level and/or abnormal digital rectal examination (DRE) and/or positive imaging and, up today, biopsy is mandatory to confirm the diagnosis. The old model consisted of Standard Biopsy (SBx), that is random sampling of the prostate gland under ultrasound guidance (TRUS), in subjects with clinical suspicion of PCa. This involves the risk of not diagnosing a high percentage of tumors (up to 30%) and of an incorrect risk stratification. Multiparametric Magnetic Resonance Imaging (mpMRI) has transformed the diagnostic pathway of PCa, not only as an imaging method for detecting suspicious lesions, but also as an intraprocedural guidance for Target Biopsy (MRI-TBx), thus bridging the diagnostic gap. Several single and multicenter randomized trials, such as PROMIS, MRI first, PRECISION and that reported by Van der Leest et al. have confirmed the superiority of the "MRI pathway", consisting of mpMRI and MRI-TBx of suspicious lesions, over the "standard pathway" of SBx in all patients with elevated PSA and/or positive DRE. MRI-TBx appears to be advantageous in reducing the overall number of biopsies performed, as well as in reducing the diagnosis of clinically insignificant disease while maintaining or improving the diagnosis of clinically significant PCa (cs-PCa). Moreover, it shows a reduction in the diagnosis of ins-PCa, and therefore, of overdiagnosis, when using MRI-TBx without sacrificing performance in the diagnosis of cs-PCa.
Conflict of interest statement
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
Figures

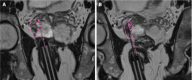





References
-
- Cohen MS, Hanley RS, Kurteva T, et al. Comparing the Gleason Prostate Biopsy and Gleason Prostatectomy Grading System: The Lahey Clinic Medical Center Experience and an International Meta-Analysis. Eur Urol. 2008. https://doi.org/10.1016/j.eururo.2008.03.049 . - PubMed
-
- Brown LC, Ahmed HU, Faria R, et al. Multiparametric MRI to improve detection of prostate cancer compared with transrectal ultrasound-guided prostate biopsy alone: The PROMIS study. Health Technol Assess (Rockv) 2018. https://doi.org/10.3310/hta22390 . - PMC - PubMed
-
- Rouvière O, Puech P, Renard-Penna R, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019. https://doi.org/10.1016/S1470–2045(18)30569-2 . - PubMed
-
- Kasivisvanathan V, Rannikko AS, Borghi M, et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767–1777. https://doi.org/10.1056/NEJMoa1801993 . - PMC - PubMed
-
- Van der Leest M, Cornel E, Israël B, et al. Head-to-head Comparison of Transrectal Ultrasound-guided Prostate Biopsy Versus Multiparametric Prostate Resonance Imaging with Subsequent Magnetic Resonance-guided Biopsy in Biopsy-naïve Men with Elevated Prostate-specific Antigen: A Large Prospective Multicenter Clinical Study. Eur Urol. 2019. https://doi.org/10.1016/j.eururo.2018.11.023 . - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

