Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids
- PMID: 33245115
- PMCID: PMC7800365
- DOI: 10.1042/BCJ20200710
Patient-derived glioblastoma stem cells transfer mitochondria through tunneling nanotubes in tumor organoids
Abstract
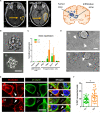
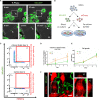
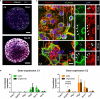
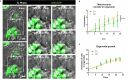
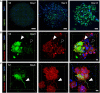
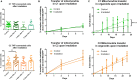
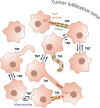
Glioblastoma (GBM) is the most aggressive brain cancer and its relapse after surgery, chemo and radiotherapy appears to be led by GBM stem cells (GSCs). Also, tumor networking and intercellular communication play a major role in driving GBM therapy-resistance. Tunneling Nanotubes (TNTs), thin membranous open-ended channels connecting distant cells, have been observed in several types of cancer, where they emerge to drive a more malignant phenotype. Here, we investigated whether GBM cells are capable to intercommunicate by TNTs. Two GBM stem-like cells (GSLCs) were obtained from the external and infiltrative zone of one GBM from one patient. We show, for the first time, that both GSLCs, grown in classical 2D culture and in 3D-tumor organoids, formed functional TNTs which allowed mitochondria transfer. In the organoid model, recapitulative of several tumor's features, we observed the formation of a network between cells constituted of both Tumor Microtubes (TMs), previously observed in vivo, and TNTs. In addition, the two GSLCs exhibited different responses to irradiation in terms of TNT induction and mitochondria transfer, although the correlation with the disease progression and therapy-resistance needs to be further addressed. Thus, TNT-based communication is active in different GSLCs derived from the external tumoral areas associated to GBM relapse, and we propose that they participate together with TMs in tumor networking.
Trial registration: ClinicalTrials.gov NCT01872221.
Keywords: cancer; cell communication; glioblastoma; stem cells; tunneling nanotubes.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures
Comment in
-
Two routes of direct intercellular communication in brain cancer.Biochem J. 2021 Mar 26;478(6):1283-1286. doi: 10.1042/BCJ20200990. Biochem J. 2021. PMID: 33755120
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

