Alveolar rhabdomyosarcoma with regional nodal involvement: Results of a combined analysis from two cooperative groups
- PMID: 33245207
- PMCID: PMC8414760
- DOI: 10.1002/pbc.28832
Alveolar rhabdomyosarcoma with regional nodal involvement: Results of a combined analysis from two cooperative groups
Abstract
Background: Treatment of children and adolescents with alveolar rhabdomyosarcoma (ARMS) and regional nodal involvement (N1) have been approached differently by North American and European cooperative groups. In order to define a better therapeutic strategy, we analyzed two studies conducted between 2005 and 2016 by the European paediatric Soft tissue sarcoma Study Group (EpSSG) and Children's Oncology Group (COG).
Methods: We retrospectively identified patients with ARMS N1 enrolled in either EpSSG RMS2005 or in COG ARST0531. Chemotherapy in RMS2005 comprised ifosfamide + vincristine + dactinomycin + doxorubicin (IVADo), IVA and maintenance (vinorelbine, cyclophosphamide); in ARST0531, it consisted of either vincristine + dactinomycin + cyclophosphamide (VAC) or VAC alternating with vincristine + irinotecan (VI). Local treatment was similar in both protocols.
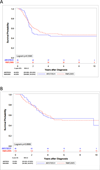
Results: The analysis of the clinical characteristics of 239 patients showed some differences between study groups: in RMS2005, advanced Intergroup Rhabdomyosarcoma Study Group (IRS) and large tumors predominated. There were no differences in outcomes between the two groups: 5-year event-free survival (EFS), 49% (95% confidence interval [CI]: 39-59) and 44% (95% CI: 30-58), and overall survival (OS), 51% (95% CI: 41-61) and 53.6% (95% CI: 40-68) in RMS2005 and ARST0531, respectively. In RMS2005, EFS of patients with FOXO1-positive tumors was significantly inferior to those with FOXO1-negative (49.3% vs 73%, P = .034). In contrast, in ARST0531, EFS of patients with FOXO1-positive tumors was 45% compared with 43.8% for those with FOXO1-negative.
Conclusions: The outcome of patients with ARMS N1 was similar in both protocols. However, patients with FOXO1 fusion-negative tumors enrolled in RMS2005 showed a significantly better outcome, suggesting that different strategies of chemotherapy may have an impact in the outcome of this subgroup of patients.
Keywords: alveolar rhabdomyosarcoma; chemotherapy; nodal involvement; prognostic factors; rhabdomyosarcoma.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Figures


References
-
- Raney RB, Anderson JR, Barr FG et al.Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: a selective review of Intergroup Rhabdomyosarcoma Study Group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol 2001;23: 215–220. - PubMed
-
- Sultan I, Qaddoumi I, Yaser S, et al.: Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: An analysis of 2,600 patients. J Clin Oncol 2009;27:3391–3397. - PubMed
-
- Carli M, Colombatti R, Oberlin O, et al.: European intergroup studies (MMT4–89 and MMT4–91) on childhood metastatic rhabdomyosarcoma: Final results and analysis of prognostic factors. J Clin Oncol 2004;22:4787–4794. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

