Points to Consider for Implementation of the ICH E17 Guideline: Learning from Past Multiregional Clinical Trials in Japan
- PMID: 33245786
- PMCID: PMC8246727
- DOI: 10.1002/cpt.2121
Points to Consider for Implementation of the ICH E17 Guideline: Learning from Past Multiregional Clinical Trials in Japan
Abstract
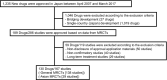
We identified the major points that are described in the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) E17 guideline but have not been considered in the past multiregional clinical trials (MRCTs) used for drug approval in Japan to elucidate potential challenges in the implementation of the ICH E17 guideline in Japan. Based on the analysis of 167 MRCTs of 130 drugs, several points, such as the same dose setting and consistency between the overall and Japanese populations, in addition to good clinical practice compliance, have been well considered in ≥ 75% of MRCTs. In contrast, the use of relevant guidelines for disease and primary end point definitions, standardization of efficacy/safety information, sample size allocation, as well as training/validation on subject selection and primary end point, have been addressed less adequately and may need to be considered when planning future MRCTs. This study provides useful information for the implementation of the ICH E17 guideline in Japan.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
The views expressed herein are the result of independent work and do not necessarily represent the views and findings of the Pharmaceuticals and Medical Devices Agency.
Figures



References
-
- Khin, N.A. et al.Regulatory and scientific issues regarding use of foreign data in support of new drug applications in the U.S: AN FDA perspective. Clin. Pharmacol. Ther. 94, 230–242 (2013). - PubMed
-
- Ueno, T. , Asahina, Y. , Tanaka, A. , Yamada, H. , Nakamura, M. & Uyama, Y. Significant differences in drug lag in clinical development among various strategies used for regulatory submissions in Japan. Clin. Pharmacol. Ther. 95, 533–541 (2014). - PubMed
-
- ICH E17 guideline: General principles for planning and design of multi‐regional clinical trials (final version) (2017). <https://database.ich.org/sites/default/files/E17EWG_Step4_2017_1116.pdf>. Accessed July 17, 2020.
-
- Ministry of Health, Labour and Welfare . Basic principles on global clinical trials (2007). <http://www.pmda.go.jp/files/000157900.pdf>. Accessed July 17, 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

