ZC3H4-a novel Cys-Cys-Cys-His-type zinc finger protein-is essential for early embryogenesis in mice†
- PMID: 33246328
- PMCID: PMC7876667
- DOI: 10.1093/biolre/ioaa215
ZC3H4-a novel Cys-Cys-Cys-His-type zinc finger protein-is essential for early embryogenesis in mice†
Abstract
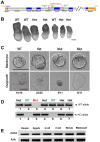
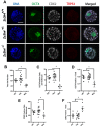
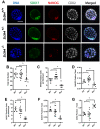
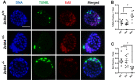
Zinc finger domains of the Cys-Cys-Cys-His (CCCH) class are evolutionarily conserved proteins that bind nucleic acids and are involved in various biological processes. Nearly 60 CCCH-type zinc finger proteins have been identified in humans and mice, most have not been functionally characterized. Here, we provide the first in vivo functional characterization of ZC3H4-a novel CCCH-type zinc finger protein. Our results show that although Zc3h4 mutant embryos exhibit normal morphology at E3.5 blastocyst stage, they cannot be recovered at E7.5 early post-gastrulation stage, suggesting implantation failure. Outgrowth assays reveal that mutant blastocysts either fail to hatch from the zona pellucida, or can hatch but do not form a typical inner cell mass colony, the source of embryonic stem cells (ESCs). Although there is no change in levels of reactive oxygen species, Zc3h4 mutants display severe DNA breaks and reduced cell proliferation. Analysis of lineage specification reveals that both epiblast and primitive endoderm lineages are compromised with severe reductions in cell number and/or specification in the mutant blastocysts. In summary, these findings demonstrate the essential role of ZC3H4 during early mammalian embryogenesis.
Keywords: Blastocyst embryo; DNA break; cell lineage specification; epiblast; inner cell mass.
© The Author(s) 2020. Published by Oxford University Press on behalf of Society for the Study of Reproduction. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Arny M, Nachtigall L, Quagliarello J. The effect of preimplantation culture conditions on murine embryo implantation and fetal development. Fertil Steril 1987; 48:861–865. - PubMed
-
- Sutherland AE, Calarco-Gillam PG. Analysis of compaction in the preimplantation mouse embryo. Dev Biol 1983; 100:328–338. - PubMed
-
- Latham KE, Solter D, Schultz RM. Activation of a two-cell stage-specific gene following transfer of heterologous nuclei into enucleated mouse embryos. Mol Reprod Dev 1991; 30:182–186. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

