Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons
- PMID: 33246331
- PMCID: PMC7880668
- DOI: 10.1093/brain/awaa376
Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons
Erratum in
-
Erratum to: Alzheimer's disease brain-derived extracellular vesicles spread tau pathology in interneurons.Brain. 2021 May 7;144(4):e42. doi: 10.1093/brain/awaa452. Brain. 2021. PMID: 33437996 Free PMC article. No abstract available.
Abstract
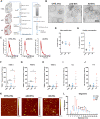
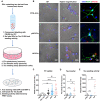
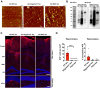
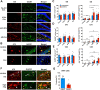
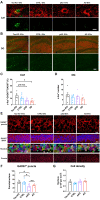
Extracellular vesicles are highly transmissible and play critical roles in the propagation of tau pathology, although the underlying mechanism remains elusive. Here, for the first time, we comprehensively characterized the physicochemical structure and pathogenic function of human brain-derived extracellular vesicles isolated from Alzheimer's disease, prodromal Alzheimer's disease, and non-demented control cases. Alzheimer's disease extracellular vesicles were significantly enriched in epitope-specific tau oligomers in comparison to prodromal Alzheimer's disease or control extracellular vesicles as determined by dot blot and atomic force microscopy. Alzheimer's disease extracellular vesicles were more efficiently internalized by murine cortical neurons, as well as more efficient in transferring and misfolding tau, than prodromal Alzheimer's disease and control extracellular vesicles in vitro. Strikingly, the inoculation of Alzheimer's disease or prodromal Alzheimer's disease extracellular vesicles containing only 300 pg of tau into the outer molecular layer of the dentate gyrus of 18-month-old C57BL/6 mice resulted in the accumulation of abnormally phosphorylated tau throughout the hippocampus by 4.5 months, whereas inoculation of an equal amount of tau from control extracellular vesicles, isolated tau oligomers, or fibrils from the same Alzheimer's disease donor showed little tau pathology. Furthermore, Alzheimer's disease extracellular vesicles induced misfolding of endogenous tau in both oligomeric and sarkosyl-insoluble forms in the hippocampal region. Unexpectedly, phosphorylated tau was primarily accumulated in glutamic acid decarboxylase 67 (GAD67) GABAergic interneurons and, to a lesser extent, glutamate receptor 2/3-positive excitatory mossy cells, showing preferential extracellular vesicle-mediated GABAergic interneuronal tau propagation. Whole-cell patch clamp recordings of CA1 pyramidal cells showed significant reduction in the amplitude of spontaneous inhibitory post-synaptic currents. This was accompanied by reductions in c-fos+ GAD67+ neurons and GAD67+ neuronal puncta surrounding pyramidal neurons in the CA1 region, confirming reduced GABAergic transmission in this region. Our study posits a novel mechanism for the spread of tau in hippocampal GABAergic interneurons via brain-derived extracellular vesicles and their subsequent neuronal dysfunction.
Keywords: Alzheimer’s disease; GABAergic interneuron; extracellular vesicle; microtubule-associated protein tau; mouse model.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures



References
-
- Arai H, Clark CM, Ewbank DC, Takase S, Higuchi S, Miura M, et al. Cerebrospinal fluid tau protein as a potential diagnostic marker in Alzheimer's disease. Neurobiol Aging 1998; 19: 125–6. - PubMed
-
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology 1992; 42: 631–9. - PubMed
-
- Baker S, Polanco JC, Gotz J. Extracellular vesicles containing P301L mutant tau accelerate pathological tau phosphorylation and oligomer formation but do not seed mature neurofibrillary tangles in ALZ17 mice. J Alzheimers Dis 2016; 54: 1207–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

