Hydroxyurea Optimization through Precision Study (HOPS): study protocol for a randomized, multicenter trial in children with sickle cell anemia
- PMID: 33246482
- PMCID: PMC7691962
- DOI: 10.1186/s13063-020-04912-z
Hydroxyurea Optimization through Precision Study (HOPS): study protocol for a randomized, multicenter trial in children with sickle cell anemia
Abstract
Background: Sickle cell disease (SCD) is a severe and devastating hematological disorder that affects over 100,000 persons in the USA and millions worldwide. Hydroxyurea is the primary disease-modifying therapy for the SCD, with proven benefits to reduce both short-term and long-term complications. Despite the well-described inter-patient variability in pharmacokinetics (PK), pharmacodynamics, and optimal dose, hydroxyurea is traditionally initiated at a weight-based dose with a subsequent conservative dose escalation strategy to avoid myelosuppression. Because the dose escalation process is time consuming and requires frequent laboratory checks, many providers default to a fixed dose, resulting in inadequate hydroxyurea exposure and suboptimal benefits for many patients. Results from a single-center trial of individualized, PK-guided dosing of hydroxyurea for children with SCD suggest that individualized dosing achieves the optimal dose more rapidly and provides superior clinical and laboratory benefits than traditional dosing strategies. However, it is not clear whether these results were due to individualized dosing, the young age that hydroxyurea treatment was initiated in the study, or both. The Hydroxyurea Optimization through Precision Study (HOPS) aims to validate the feasibility and benefits of this PK-guided dosing approach in a multi-center trial.
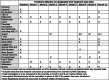
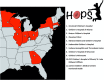
Methods: HOPS is a randomized, multicenter trial comparing standard vs. PK-guided dosing for children with SCD as they initiate hydroxyurea therapy. Participants (ages 6 months through 21 years), recruited from 11 pediatric sickle cell centers across the USA, are randomized to receive hydroxyurea either using a starting dose of 20 mg/kg/day (Standard Arm) or a PK-guided dose (Alternative Arm). PK data will be collected using a novel sparse microsampling approach requiring only 10 μL of blood collected at 3 time-points over 3 h. A protocol-guided strategy more aggressive protocols is then used to guide dose escalations and reductions in both arms following initiation of hydroxyurea. The primary endpoint is the mean %HbF after 6 months of hydroxyurea.
Discussion: HOPS will answer important questions about the clinical feasibility, benefits, and safety of PK-guided dosing of hydroxyurea for children with SCD with potential to change the treatment paradigm from a standard weight-based approach to one that safely and effectively optimize the laboratory and clinical response.
Trial registration: ClinicalTrials.gov NCT03789591 . Registered on 28 December 2018.
Keywords: Hydroxyurea; Pediatrics; Pharmacokinetics; Sickle cell anemia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

