Oleanolic acid ameliorates intestinal alterations associated with EAE
- PMID: 33246492
- PMCID: PMC7697371
- DOI: 10.1186/s12974-020-02042-6
Oleanolic acid ameliorates intestinal alterations associated with EAE
Abstract
Background: Multiple sclerosis (MS) is a chronic demyelinating autoimmune disease affecting the CNS. Recent studies have indicated that intestinal alterations play key pathogenic roles in the development of autoimmune diseases, including MS. The triterpene oleanolic acid (OA), due to its anti-inflammatory properties, has shown to beneficially influence the severity of the experimental autoimmune encephalomyelitis (EAE), a preclinical model of MS. We herein investigate EAE-associated gut intestinal dysfunction and the effect of OA treatment.
Methods: Mice with MOG35-55-induced EAE were treated with OA or vehicle from immunization day and were daily analyzed for clinical deficit. We performed molecular and histological analysis in serum and intestinal tissues to measure oxidative and inflammatory responses. We used Caco-2 and HT29-MTX-E12 cells to elucidate OA in vitro effects.
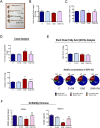
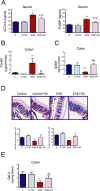
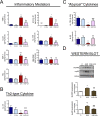
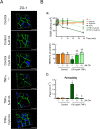
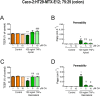
Results: We found that OA protected from EAE-induced changes in intestinal permeability and preserved the mucin-containing goblet cells along the intestinal tract. Serum levels of the markers for intestinal barrier damage iFABP and monocyte activation sCD14 were consistently and significantly reduced in OA-treated EAE mice. Beneficial OA effects also included a decrease of pro-inflammatory mediators both in serum and colonic tissue of treated-EAE mice. Moreover, the levels of some immunoregulatory cytokines, the neurotrophic factor GDNF, and the gastrointestinal hormone motilin were preserved in OA-treated EAE mice. Regarding oxidative stress, OA treatment prevented lipid peroxidation and superoxide anion accumulation in intestinal tissue, while inducing the expression of the ROS scavenger Sestrin-3. Furthermore, short-chain fatty acids (SCFA) quantification in the cecal content showed that OA reduced the high iso-valeric acid concentrations detected in EAE-mice. Lastly, using in vitro cell models which mimic the intestinal epithelium, we verified that OA protected against intestinal barrier dysfunction induced by injurious agents produced in both EAE and MS.
Conclusion: These findings reveal that OA ameliorates the gut dysfunction found in EAE mice. OA normalizes the levels of gut mucosal dysfunction markers, as well as the pro- and anti-inflammatory immune bias during EAE, thus reinforcing the idea that OA is a beneficial compound for treating EAE and suggesting that OA may be an interesting candidate to be explored for the treatment of human MS.
Keywords: Cytokines; EAE; Immune markers; Inflammation; Intestinal dysfunction; Mucins; Multiple sclerosis; Oleanolic acid; Oxidative stress; Triterpenes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Calabresi PA. Diagnosis and management of multiple sclerosis. Am Fam Physician. 2004;70:1935–1944. - PubMed
-
- Buscarinu MC, Cerasoli B, Annibali V, Policano C, Lionetto L, Capi M, Mechelli R, Romano S, Fornasiero A, Mattei G, Piras E, Angelini DF, Battistini L, Simmaco M, Umeton R, Salvetti M, Ristori G. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: A pilot study. Mult Scler. 2017;23:442–446. doi: 10.1177/1352458516652498. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

