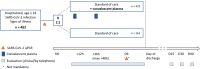
A randomized, multicentre, open-label phase II proof-of-concept trial investigating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19: the Donated Antibodies Working against nCoV (DAWn-Plasma) trial
- PMID: 33246499
- PMCID: PMC7691949
- DOI: 10.1186/s13063-020-04876-0
A randomized, multicentre, open-label phase II proof-of-concept trial investigating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19: the Donated Antibodies Working against nCoV (DAWn-Plasma) trial
Erratum in
-
Correction to: A randomized, multicentre, open-label phase II proof-of-concept trial investigating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19: the Donated Antibodies Working against nCoV (DAWn-Plasma) trial.Trials. 2020 Dec 14;21(1):1024. doi: 10.1186/s13063-020-04947-2. Trials. 2020. PMID: 33317581 Free PMC article. No abstract available.
Abstract
Background: The COVID-19 pandemic has imposed an enormous burden on health care systems around the world. In the past, the administration of convalescent plasma of patients having recovered from SARS and severe influenza to patients actively having the disease showed promising effects on mortality and appeared safe. Whether or not this also holds true for the novel SARS-CoV-2 virus is currently unknown.
Methods: DAWn-Plasma is a multicentre nation-wide, randomized, open-label, phase II proof-of-concept clinical trial, evaluating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19 in Belgium. Patients hospitalized with a confirmed diagnosis of COVID-19 are eligible when they are symptomatic (i.e. clinical or radiological signs) and have been diagnosed with COVID-19 in the 72 h before study inclusion through a PCR (nasal/nasopharyngeal swab or bronchoalveolar lavage) or a chest-CT scan showing features compatible with COVID-19 in the absence of an alternative diagnosis. Patients are randomized in a 2:1 ratio to either standard of care and convalescent plasma (active treatment group) or standard of care only. The active treatment group receives 2 units of 200 to 250 mL of convalescent plasma within 12 h after randomization, with a second administration of 2 units 24 to 36 h after ending the first administration. The trial aims to include 483 patients and will recruit from 25 centres across Belgium. The primary endpoint is the proportion of patients that require mechanical ventilation or have died at day 15. The main secondary endpoints are clinical status on day 15 and day 30 after randomization, as defined by the WHO Progression 10-point ordinal scale, and safety of the administration of convalescent plasma.
Discussion: This trial will either provide support or discourage the use of convalescent plasma as an early intervention for the treatment of hospitalized patients with COVID-19 infection.
Trial registration: ClinicalTrials.gov NCT04429854 . Registered on 12 June 2020 - Retrospectively registered.
Keywords: Antibodies; COVID-19; Convalescent plasma; Immunity; SARS-CoV-2.
Conflict of interest statement
The authors declare that they have no competing interests.
References
-
- Mair-Jenkins J, Saavedra-Campos M, Kenneth Baillie J, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. 2014. 10.1093/infdis/jiu396. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous