The Role of Theranostics in Prostate Cancer
- PMID: 33246638
- PMCID: PMC8391014
- DOI: 10.1016/j.semradonc.2020.07.004
The Role of Theranostics in Prostate Cancer
Abstract
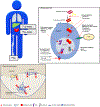
Theranostics in men with metastatic castration-resistant prostate cancer (mCRPC) has been developed to target bone and the tumor itself. Currently, bone-directed targeted alpha therapy with radium-223 (223Ra) is the only theranostic agent proven to prolong survival in men with mCRPC who have symptomatic bone metastases and no known visceral metastases. The clinical utility and therapeutic success of 223Ra has encouraged the development of other tumor-targeting theranostic agents in mCRPC, primarily targeting prostate-specific membrane antigen (PSMA) with radioligand therapy (RLT). There is increasing evidence of promising response rates and a low toxicity profile with 177Lu-labeled PSMA RLT in patients with mCRPC. A phase III randomized study of 177Lu-labeled PSMA RLT has completed accrual and is awaiting results as to whether the drug improves radiographic progression-free survival and overall survival in men with mCRPC receiving standard of care treatments. Additional early clinical trials are investigating the role of tumor-directed targeted alpha therapy with radiotracers such as 225Ac. In this article, we review the current status of theranostics in prostate cancer, discussing the challenges and opportunities of combination therapies with more conventional agents such as androgen receptor inhibitors, cytotoxic chemotherapy, and immunotherapy.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Conflicts of Interest: None.
Figures
References
-
- Allemani C, Matsuda T, Di Carlo V, et al.: Global surveillance of trends in cancer survival 2000−14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 391:1023–1075, 2018. 10.1016/S0140-6736(17)33326-3 - DOI - PMC - PubMed
-
- Prostate Cancer Statistics | World Cancer Research Fund. Available at: https://www.wcrf.org/dietandcancer/cancer-trends/prostate-cancer-statistics. Accessed February 13, 2020.
-
- James ND, Sydes MR, Clarke NW, et al.: Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet 387:1163–1177, 2016. 10.1016/S0140-6736(15)01037-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

