Long-term azithromycin use is not associated with QT prolongation in children with cystic fibrosis
- PMID: 33246911
- PMCID: PMC8009808
- DOI: 10.1016/j.jcf.2020.11.005
Long-term azithromycin use is not associated with QT prolongation in children with cystic fibrosis
Abstract
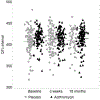
Chronic Azithromycin (AZM) is a common treatment for lung infection. Among adults at risk of cardiac events, AZM use has been associated with cardiovascular harm. We assessed cardiovascular safety of AZM among children with CF, as a secondary analysis of a placebo-controlled, clinical trial, in which study drug was taken thrice-weekly for a planned 18 months. Safety assessments using electrocardiogram (ECG) occurred at study enrollment, and then after 3 weeks and 18 months of participation. Among 221 study participants with a median of 18 months follow-up, increased corrected QT interval (QTc) of ≥30 msec was rare, at 3.4 occurrences per 100 person-years; and incidence of QTc prolongation was no higher in the AZM arm than the placebo arm (1.8 versus 5.4 per 100 person-years). No persons experienced QTc intervals above 500 msec. Long-term chronic AZM use was not associated with increased QT prolongation.
Keywords: Azithromycin; Cystic fibrosis; Pulmonary exacerbation; QT prolongation.
Copyright © 2020 European Cystic Fibrosis Society. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Shaffer D, et al. , Concomitant risk factors in reports of torsades de pointes associated with macrolide use: review of the United States Food and Drug Administration Adverse Event Reporting System. Clin Infect Dis, 2002. 35(2): p. 197–200. - PubMed
-
- Food and Drug Administration, FDA Drug Safety Communication: Azithromycin (Zithromax or Zmax) and the risk of potentially fatal heart rhythms. 2013: https://www.fda.gov/media/85787/download.
-
- Goldenberg I, Moss AJ, and Zareba W, QT interval: How to measure it and what is “normal”. Journal of Cardiovascular Electrophysiology, 2006. 17(3): p. 333–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical