Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates
- PMID: 33247108
- PMCID: PMC7699647
- DOI: 10.1038/s41467-020-19843-1
Nucleocapsid protein of SARS-CoV-2 phase separates into RNA-rich polymerase-containing condensates
Abstract
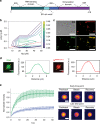
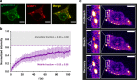
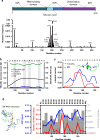
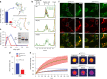
The etiologic agent of the Covid-19 pandemic is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The viral membrane of SARS-CoV-2 surrounds a helical nucleocapsid in which the viral genome is encapsulated by the nucleocapsid protein. The nucleocapsid protein of SARS-CoV-2 is produced at high levels within infected cells, enhances the efficiency of viral RNA transcription, and is essential for viral replication. Here, we show that RNA induces cooperative liquid-liquid phase separation of the SARS-CoV-2 nucleocapsid protein. In agreement with its ability to phase separate in vitro, we show that the protein associates in cells with stress granules, cytoplasmic RNA/protein granules that form through liquid-liquid phase separation and are modulated by viruses to maximize replication efficiency. Liquid-liquid phase separation generates high-density protein/RNA condensates that recruit the RNA-dependent RNA polymerase complex of SARS-CoV-2 providing a mechanism for efficient transcription of viral RNA. Inhibition of RNA-induced phase separation of the nucleocapsid protein by small molecules or biologics thus can interfere with a key step in the SARS-CoV-2 replication cycle.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Virological.org. Novel 2019 Coronavirus Genome. Available online: http://virological.org/t/novel-2019-coronavirus-genome/319. (2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

