The PAR2 inhibitor I-287 selectively targets Gαq and Gα12/13 signaling and has anti-inflammatory effects
- PMID: 33247181
- PMCID: PMC7695697
- DOI: 10.1038/s42003-020-01453-8
The PAR2 inhibitor I-287 selectively targets Gαq and Gα12/13 signaling and has anti-inflammatory effects
Abstract
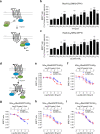
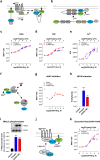
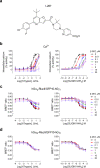
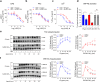
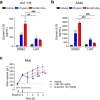
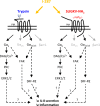
Protease-activated receptor-2 (PAR2) is involved in inflammatory responses and pain, therefore representing a promising therapeutic target for the treatment of immune-mediated inflammatory diseases. However, as for other GPCRs, PAR2 can activate multiple signaling pathways and those involved in inflammatory responses remain poorly defined. Here, we describe a new selective and potent PAR2 inhibitor (I-287) that shows functional selectivity by acting as a negative allosteric regulator on Gαq and Gα12/13 activity and their downstream effectors, while having no effect on Gi/o signaling and βarrestin2 engagement. Such selective inhibition of only a subset of the pathways engaged by PAR2 was found to be sufficient to block inflammation in vivo. In addition to unraveling the PAR2 signaling pathways involved in the pro-inflammatory response, our study opens the path toward the development of new functionally selective drugs with reduced liabilities that could arise from blocking all the signaling activities controlled by the receptor.
Conflict of interest statement
M.S. and F.G. were employees of IRIC at the Université de Montréal during their involvement in this work and are now employees of Domain Therapeutics N.A. to which some of the biosensors used in the present study were licensed for commercial purposes. C.S., Y.B., and C.E.S. were employees of Vertex Pharmaceuticals (Canada) during their involvement in this work and are now employees of Paraza Pharma, Inc., AdMare BioInnovations, and Ra Pharmaceuticals, respectively. Y.B. is CSO at AdMare BioInnovations and the Chair of the BOD of Domain Therapeutics. J.A.M. holds stocks of Vertex Pharmaceuticals. C.E.S. holds stocks of UCB. M.B. is the president of Domain Therapeutics SAB. C.S. and C.E.S. are among the inventers who filed a patent application related to the compound I-287. C.L.G. and M.B. are among the inventers who filed patent applications related to the biosensors used in this work. These biosensors were licensed to Domain Therapeutics for commercial use. C.A., S.G., and L.G. have no competing interests to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

