Brain volumetric deficits in MAPT mutation carriers: a multisite study
- PMID: 33247623
- PMCID: PMC7818091
- DOI: 10.1002/acn3.51249
Brain volumetric deficits in MAPT mutation carriers: a multisite study
Abstract
Objective: MAPT mutations typically cause behavioral variant frontotemporal dementia with or without parkinsonism. Previous studies have shown that symptomatic MAPT mutation carriers have frontotemporal atrophy, yet studies have shown mixed results as to whether presymptomatic carriers have low gray matter volumes. To elucidate whether presymptomatic carriers have lower structural brain volumes within regions atrophied during the symptomatic phase, we studied a large cohort of MAPT mutation carriers using a voxelwise approach.
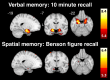
Methods: We studied 22 symptomatic carriers (age 54.7 ± 9.1, 13 female) and 43 presymptomatic carriers (age 39.2 ± 10.4, 21 female). Symptomatic carriers' clinical syndromes included: behavioral variant frontotemporal dementia (18), an amnestic dementia syndrome (2), Parkinson's disease (1), and mild cognitive impairment (1). We performed voxel-based morphometry on T1 images and assessed brain volumetrics by clinical subgroup, age, and mutation subtype.
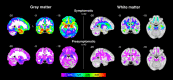
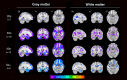
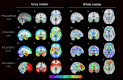
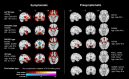
Results: Symptomatic carriers showed gray matter atrophy in bilateral frontotemporal cortex, insula, and striatum, and white matter atrophy in bilateral corpus callosum and uncinate fasciculus. Approximately 20% of presymptomatic carriers had low gray matter volumes in bilateral hippocampus, amygdala, and lateral temporal cortex. Within these regions, low gray matter volumes emerged in a subset of presymptomatic carriers as early as their thirties. Low white matter volumes arose infrequently among presymptomatic carriers.
Interpretation: A subset of presymptomatic MAPT mutation carriers showed low volumes in mesial temporal lobe, the region ubiquitously atrophied in all symptomatic carriers. With each decade of age, an increasing percentage of presymptomatic carriers showed low mesial temporal volume, suggestive of early neurodegeneration.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
SAC, TMF, LCJ, JD, SS, LZ, VES, JSY, WWS, JMP, DHG, HWH, LKF, DEB, MG, YMB, JCF, TF, RHG, DJI, AMK, JHK, WAK, MIL, MFM, AYP, RR, EMR, MCT, NAT, AV, SW, BW, JCVS, and SEL had no disclosures. AMS received research support from the Larry H. Hillblom foundation and support from the NIH. HJR received research support from Biogen Pharmaceuticals, has consulting agreements with Wave Neuroscience and Ionis Pharmaceuticals, and receives research support from the NIH. BFB served as an investigator for clinical trials sponsored by GE Healthcare and Axovant. He received royalties from the publication of a book entitled Behavioral Neurology of Dementia (Cambridge Medicine, 2009, 2017). He served on the Scientific Advisory Board of the Tau Consortium. He received research support from the NIH, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program and the Little Family Foundation. ALB received research support from the NIH, the Tau Research Consortium, the Association for Frontotemporal Degeneration, Bluefield Project to Cure Frontotemporal Dementia, Corticobasal Degeneration Solutions, the Alzheimer's Drug Discovery Foundation, and the Alzheimer's Association. He served as a consultant for Aeton, Abbvie, Alector, Amgen, Arkuda, Ionis, Iperian, Janssen, Merck, Novartis, Samumed, Toyama and UCB, and received research support from Avid, Biogen, BMS, C2N, Cortice, Eli Lilly, Forum, Genentech, Janssen, Novartis, Pfizer, Roche, and TauRx. GC, BCD, KF, JAF, EDH, BLM, AWT received research support from the NIH. HHF reported grants from Toyama Pharmaceuticals, Biohaven Pharmaceuticals, Annovis Bio (QR Pharma) and Vivoryon (Probiodrug). He also reported service agreements through UCSD for consulting with Eisai, Merck, Tau RX, Samus, Arkuda, Samumed, Axon Neurosciences, Roche/Genentech Pharmaceuticals for DMC and DSMB activities; Tau Consortium for Scientific Advisory Board. He reported travel expenses from Vivoryon, Axon Neurosciences and Alion Pharmaceuticals and speaker fees to UCSD from World Events Forum, Optum Health, and Medscape. NG had participated or is currently participating in clinical trials of antidementia drugs sponsored by the following companies: Bristol Myers Squibb, Eli Lilly/Avid Radiopharmaceuticals, Janssen Immunotherapy, Novartis, Pfizer, Wyeth, SNIFF (The Study of Nasal Insulin to Fight Forgetfulness) study, and A4 (The Anti‐Amyloid Treatment in Asymptomatic Alzheimer's Disease) trial. She received research support from Tau Consortium and Association for Frontotemporal Dementia and is funded by the NIH. NRGR received royalties from UpToDate and had participated in multicenter therapy studies sponsored by Biogen, TauRx, AbbVie, Novartis, and Lilly. He received research support from the NIH. GYRH had served as an investigator for clinical trials sponsored by AstraZeneca, Eli Lilly, and Roche/Genentech. He received research support from the Canadian Institutes of Health Research and the Alzheimer Society of British Columbia. KK served on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc.; data monitoring boards of Pfizer and Janssen Alzheimer Immunotherapy; research support from the Avid Radiopharmaceuticals, Eli Lilly, the Alzheimer's Drug Discovery Foundation, and the NIH. DIK served as a consultant to Axovant, Abbvie, Janssen Research and Development, and Takeda. He served on the advisory board of Grifols, and received research support from Axovant, Janssen Research and Development, Navidea Biopharmaceuticals, and Acadia. DSK served on the DSMB of the DIAN‐TU study, is a site PI for clinical trials sponsored by Biogen, Lilly and the University of Southern California, and is funded by the NIH. JK had provided expert witness testimony for Teva Pharmaceuticals in Forest Laboratories Inc. et al. v. Teva Pharmaceuticals USA, Inc., Case Nos. 1:14‐cv‐00121 and 1:14‐cv‐00686 (D. Del. filed Jan. 31, 2014 and May 30, 2014) regarding the drug Memantine; for Apotex/HEC/Ezra in Novartis AG et al. v. Apotex Inc., No. 1:15‐cv‐975 (D. Del. filed Oct. 26, 2015, regarding the drug Fingolimod. He had also given testimony on behalf of Puma Biotechnology in Hsingching Hsu et al., vs. Puma Biotechnology, INC., et al. 2018 regarding the drug neratinib. He received research support from the NIH. IL received research support from the NIH, Parkinson Study Group, Parkinson Foundation, Michael J Fox Foundation, AVID Pharmaceuticals, C2N Diagnostics/Abbvie, and Bristol‐Myers Squibb. She was a member of the Biogen and Bristol‐Myers Squibb Advisory Boards, Biotie/Parkinson Study Group Medical Advisory Board, and consultant for Toyama Pharmaceuticals. She received salary from the University of California San Diego and as Editor in Frontiers in Neurology. IRAM received research funding from the Canadian Institutes of Health Research. S.M.M. served as an investigator for clinical trials sponsored by AbbVie, Allon Therapeutics, Biogen, Bristol‐Myers Squibb, C2N Diagnostics, Eisai Inc., Eli Lilly and Co., Genentech, Janssen Pharmaceuticals, Medivation, Merck, Navidea Biopharmaceuticals, Novartis, Pfizer, and TauRx Therapeutics. He received research support from the NIH. CUO received research funding from the NIH, the CIHR, and Biogen, Inc. He was also supported by the Jane Tanger Black Fund for Young‐Onset Dementias, the Nancy H. Hall Fund for Geriatric Psychiatry, and the gift from Joseph Trovato. EDR received research support from the NIH, Bluefield Project to Cure Frontotemporal Dementia, Alzheimer's Association, BrightFocus Foundation, Biogen, Alector, and owns intellectual property related to tau. ZKW was supported by the NIH, Mayo Clinic Center for Regenerative Medicine, the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch, the Sol Goldman Charitable Trust, and Donald G. and Jodi P. Heeringa. He has also received grant funding support from Allergan, Inc. (educational grant), and Abbvie (medication trials).
Figures
References
-
- Ghetti B, Wszolek ZK, Boeve BF, et al. Frontotemporal Dementia and Parkinsonism Linked to Chromosome 17 Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Ltd: John Wiley & Sons, 2011:110–134. 10.1002/9781444341256.ch14. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- P30 AG066507/AG/NIA NIH HHS/United States
- R01 AG058233/AG/NIA NIH HHS/United States
- K24 AG045333/AG/NIA NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- P50 AG023501/NH/NIH HHS/United States
- 2R01AG038791/NH/NIH HHS/United States
- P30 AG062429/AG/NIA NIH HHS/United States
- U24AG21886/AG/NIA NIH HHS/United States
- UH3 NS103870/NS/NINDS NIH HHS/United States
- P01 AG066597/AG/NIA NIH HHS/United States
- R01 AG057204/AG/NIA NIH HHS/United States
- U01 AG045390/NH/NIH HHS/United States
- K08 AG052648/AG/NIA NIH HHS/United States
- U54 NS092089/NH/NIH HHS/United States
- R01 AG062268/AG/NIA NIH HHS/United States
- U01 AG045390/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- R01 AG026938/AG/NIA NIH HHS/United States
- K23AG039414/NH/NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- R01 NS076837/NS/NINDS NIH HHS/United States
- RC1 AG035610/AG/NIA NIH HHS/United States
- L30 AG064696/AG/NIA NIH HHS/United States
- K23 AG061253/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- U24 AG072122/AG/NIA NIH HHS/United States
- P01 AG019724/NH/NIH HHS/United States
- P30 AG062421/AG/NIA NIH HHS/United States
- R35 NS097261/NS/NINDS NIH HHS/United States
- R01 NS076837/NH/NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- P50 AG008702/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- R01 AG052496/AG/NIA NIH HHS/United States
- K23 AG059891/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- P30 AG066462/AG/NIA NIH HHS/United States
- UG3 NS103870/NH/NIH HHS/United States
- P30 AG066530/AG/NIA NIH HHS/United States
- K23 AG039414/AG/NIA NIH HHS/United States
- R01 MH120794/MH/NIMH NIH HHS/United States
- AG035610/NH/NIH HHS/United States
- R01 AG26938/NH/NIH HHS/United States
- R35 NS097261/NH/NIH HHS/United States
- UG3 NS103870/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
