Oral berotralstat for the prophylaxis of hereditary angioedema attacks in patients in Japan: A phase 3 randomized trial
- PMID: 33247955
- PMCID: PMC8247297
- DOI: 10.1111/all.14670
Oral berotralstat for the prophylaxis of hereditary angioedema attacks in patients in Japan: A phase 3 randomized trial
Abstract
Background: With no approved treatments in Japan for the prevention of hereditary angioedema (HAE) attacks, there is a significant unmet need for long-term prophylactic therapies for Japanese patients with HAE. Berotralstat (BCX7353) is an oral, once-daily, highly selective inhibitor of plasma kallikrein in development for prophylaxis of angioedema attacks in HAE patients.
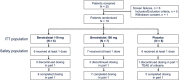
Methods: APeX-J is a phase 3, randomized, double-blind, placebo-controlled, parallel-group, 3-part trial conducted in Japan (University Hospital Medical Information Network identifier, UMIN000034869; ClinicalTrials.gov identifier, NCT03873116). Patients with a clinical diagnosis of type 1 or 2 HAE underwent a prospective run-in period of 56 days to determine eligibility, allowing enrollment of those with ≥2 expert-confirmed angioedema attacks. Patients were randomly assigned (1:1:1) and stratified by baseline attack rate (≥2 vs. <2 expert-confirmed attacks/month between screening and randomization) to receive once-daily berotralstat 110 mg, berotralstat 150 mg, or placebo. The primary endpoint was the rate of expert-confirmed angioedema attacks during dosing in the 24-week treatment period.
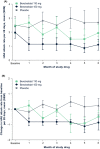
Results: Nineteen patients were randomized to receive once-daily berotralstat 110 mg (n = 6), berotralstat 150 mg (n = 7), or placebo (n = 6). Treatment with berotralstat 150 mg significantly reduced HAE attacks relative to placebo (1.11 vs. 2.18 attacks/month, p = .003). The most frequently reported treatment-emergent adverse events (TEAEs) in berotralstat-treated patients (n = 13) were nasopharyngitis (n = 4, 31%), abdominal pain, cough, diarrhea, and pyrexia (n = 2 each, 15%).
Conclusions: Orally administered, once-daily berotralstat 150 mg significantly reduced the frequency of HAE attacks and was safe and well tolerated, supporting its use as a prophylactic therapy in patients with type 1 or 2 HAE in Japan.
Keywords: Japan; berotralstat; hereditary angioedema; kallikrein inhibitor; prophylaxis.
© 2020 The Authors. Allergy published by European Academy of Allergy and Clinical Immunology and John Wiley & Sons Ltd.
Conflict of interest statement
DH reports speaker fees from CSL Behring, Kyowa Kirin, Otsuka, Shire, and Takeda outside the submitted work. GC, MC, SCM, SMD, EN, SVD, LR, JB, HI, PC, and WPS are employees of BioCryst Pharmaceuticals. GC, EN, LR, and WPS hold stock options in BioCryst Pharmaceuticals. MH reports personal fees from BioCryst Pharmaceuticals during the conduct of the study; personal fees from Shire/Takeda; and grants and personal fees from CSL Behring, outside the submitted work; and a grant of the Ministry of Health, Labour and Welfare of Japan. IO, YSuzuki, TF, KK, EM, SM, OI, YSasaki, and MT have nothing to disclose.
Figures

References
-
- Zuraw BL, Christiansen SC. HAE pathophysiology and underlying mechanisms. Clin Rev Allergy Immunol. 2016;51(2):216‐229. - PubMed
-
- Kaplan AP, Joseph K. The bradykinin‐forming cascade and its role in hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104(3):193‐204. - PubMed
-
- Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267‐274. - PubMed
-
- Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1‐INH deficiency. J Allergy Clin Immunol. 2012;130(3):692‐697. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

