Systems and Clinical Pharmacology of COVID-19 Therapeutic Candidates: A Clinical and Translational Medicine Perspective
- PMID: 33248057
- PMCID: PMC7689305
- DOI: 10.1016/j.xphs.2020.11.019
Systems and Clinical Pharmacology of COVID-19 Therapeutic Candidates: A Clinical and Translational Medicine Perspective
Abstract
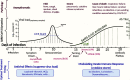
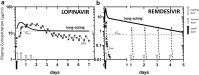
Over 50 million people have been infected with the SARS-CoV-2 virus, while around 1 million have died due to COVID-19 disease progression. COVID-19 presents flu-like symptoms that can escalate, in about 7-10 days from onset, into a cytokine storm causing respiratory failure and death. Although social distancing reduces transmissibility, COVID-19 vaccines and therapeutics are essential to regain socioeconomic normalcy. Even if effective and safe vaccines are found, pharmacological interventions are still needed to limit disease severity and mortality. Integrating current knowledge and drug candidates (approved drugs for repositioning among >35 candidates) undergoing clinical studies (>3000 registered in ClinicalTrials.gov), we employed Systems Pharmacology approaches to project how antivirals and immunoregulatory agents could be optimally evaluated for use. Antivirals are likely to be effective only at the early stage of infection, soon after exposure and before hospitalization, while immunomodulatory agents should be effective in the later-stage cytokine storm. As current antiviral candidates are administered in hospitals over 5-7 days, a long-acting combination that targets multiple SARS-CoV-2 lifecycle steps may provide a long-lasting, single-dose treatment in outpatient settings. Long-acting therapeutics may still be needed even when vaccines become available as vaccines are likely to be approved based on a 50% efficacy target.
Keywords: COVID-19; Long-acting; Modeling; Pharmacokinetics; SARS-CoV-2; Systems pharmacology.
Copyright © 2020 American Pharmacists Association®. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Mallapaty S. The coronavirus is most deadly if you are older and male — new data reveal the risks. Nature. 2020;585(7823):16–17. - PubMed
-
- Selen A., Müllertz A., Kesisoglou F. Integrated multi-stakeholder systems thinking strategy: decision-making with biopharmaceutics risk assessment roadmap (BioRAM) to optimize clinical performance of drug products. AAPS J. 2020;22(5):97. - PubMed
-
- Gilead Sciences Drug Approval Package: VEKLURY (remdesivir) injection, for intravenous use. 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/214787Orig1s00...
-
- World Health Organization Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
-
- U.S. Center for disease Control and Prevention . CDC; 2009. H1N1 - Summary of Progress since 2009.https://www.cdc.gov/flu/pandemic-resources/h1n1-summary.htm
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

