In vivo analysis of the spontaneous firing of cerebellar Purkinje cells in awake transgenic mice that model spinocerebellar ataxia type 2
- PMID: 33248384
- PMCID: PMC7855958
- DOI: 10.1016/j.ceca.2020.102319
In vivo analysis of the spontaneous firing of cerebellar Purkinje cells in awake transgenic mice that model spinocerebellar ataxia type 2
Abstract
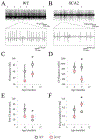
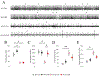
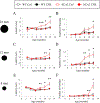
Cerebellar Purkinje cells (PCs) fire spontaneously in a tonic mode, although the precision of this pacemaking activity is disturbed in many abnormal conditions involving cerebellar atrophy, such as many spinocerebellar ataxias (SCAs). In our previous studies we used the single-unit extracellular recording method to analyze spontaneous PC firing in vivo in the anesthetized SCA2-58Q transgenic mice. We realized that PCs from aging SCA2-58Q mice fire much less regularly compared to PCs from their wild type (WT) littermates and this abnormal activity can be reversed with an intraperitoneal (i. p.) injection of SK channel-positive modulator chlorzoxazone (CHZ). Here we used the same single-unit extracellular recording method to analyze the spontaneous firing in vivo in awake SCA2-58Q transgenic mice. For this purpose, we used the Mobile HomeCage (Neurotar, Finland) floating platform to immobilize the experimental animal's head during the recording sessions. We discovered that generally PCs from awake animals fired much more frequently and much less regularly than previously observed PCs from anesthetized animals. In vivo recordings from awake SCA2/WT mice revealed that complex spikes, which are generated by PCs in reply to the excitation coming by climbing fibers, as well as simple spikes, were much less frequent in SCA2 mice compared to their WT littermates. To test the effect of the SK channel positive modulation on the PCs firing activity in awake SCA2 mice and also the effect on their motor coordination, we started the CHZ trial in these mice. We discovered that the long-term i. p. injections of CHZ did not affect the spike generation in SCA2-58Q mice, however, they did recover the precision of this spontaneous pacemaking activity. Furthermore, we also showed that treatment with CHZ alleviated the age-dependent motor impairment in SCA2-58Q mice. We propose that the lack of precision in PC spike generation might be a key cause for the progression of ataxic symptoms in different SCAs and that the activation of calcium-activated potassium channels, including SK channels, can be used as a potential way to treat SCAs on the physiological level of the disease.
Keywords: Cerebellum; Chlorzoxazone; In vivo electrophysiology; SK channels; Spinocerebellar ataxia; Transgenic mice.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement:
The authors declare that they have no conflict of interest related to this mansucript.
Figures
Similar articles
-
A chlorzoxazone-folic acid combination improves cognitive affective decline in SCA2-58Q mice.Sci Rep. 2023 Aug 3;13(1):12588. doi: 10.1038/s41598-023-39331-y. Sci Rep. 2023. PMID: 37537226 Free PMC article.
-
Electrophysiological Studies Support Utility of Positive Modulators of SK Channels for the Treatment of Spinocerebellar Ataxia Type 2.Cerebellum. 2022 Oct;21(5):742-749. doi: 10.1007/s12311-021-01349-1. Epub 2022 Jan 3. Cerebellum. 2022. PMID: 34978024 Review.
-
In vivo analysis of cerebellar Purkinje cell activity in SCA2 transgenic mouse model.J Neurophysiol. 2016 Jun 1;115(6):2840-51. doi: 10.1152/jn.00913.2015. Epub 2016 Mar 16. J Neurophysiol. 2016. PMID: 26984424 Free PMC article.
-
In Vivo Analysis of the Climbing Fiber-Purkinje Cell Circuit in SCA2-58Q Transgenic Mouse Model.Cerebellum. 2018 Oct;17(5):590-600. doi: 10.1007/s12311-018-0951-4. Cerebellum. 2018. PMID: 29876801 Free PMC article.
-
Deranged calcium signaling in Purkinje cells and pathogenesis in spinocerebellar ataxia 2 (SCA2) and other ataxias.Cerebellum. 2012 Sep;11(3):630-9. doi: 10.1007/s12311-010-0182-9. Cerebellum. 2012. PMID: 20480274 Free PMC article. Review.
Cited by
-
A chlorzoxazone-folic acid combination improves cognitive affective decline in SCA2-58Q mice.Sci Rep. 2023 Aug 3;13(1):12588. doi: 10.1038/s41598-023-39331-y. Sci Rep. 2023. PMID: 37537226 Free PMC article.
-
Electrophysiological Studies Support Utility of Positive Modulators of SK Channels for the Treatment of Spinocerebellar Ataxia Type 2.Cerebellum. 2022 Oct;21(5):742-749. doi: 10.1007/s12311-021-01349-1. Epub 2022 Jan 3. Cerebellum. 2022. PMID: 34978024 Review.
-
Cognitive Decline and Mood Alterations in the Mouse Model of Spinocerebellar Ataxia Type 2.Cerebellum. 2024 Feb;23(1):145-161. doi: 10.1007/s12311-023-01520-w. Epub 2023 Jan 21. Cerebellum. 2024. PMID: 36680704
-
A combination of chlorzoxazone and folic acid improves recognition memory, anxiety and depression in SCA3-84Q mice.Hum Mol Genet. 2024 Aug 6;33(16):1406-1419. doi: 10.1093/hmg/ddae079. Hum Mol Genet. 2024. PMID: 38727562 Free PMC article.
-
Structure-Activity Relationship Study of Subtype-Selective Positive Modulators of KCa2 Channels.J Med Chem. 2022 Jan 13;65(1):303-322. doi: 10.1021/acs.jmedchem.1c01473. Epub 2021 Dec 28. J Med Chem. 2022. PMID: 34962403 Free PMC article.
References
-
- Magana JJ, Velazquez-Perez L, Cisneros B. Spinocerebellar ataxia type 2: clinical presentation, molecular mechanisms, and therapeutic perspectives. Molecular neurobiology. 2013;47(1):90–104. - PubMed
-
- Scoles DR, Pulst SM. Spinocerebellar Ataxia Type 2. Advances in experimental medicine and biology. 2018;1049:175–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

