The efficacy and safety of adding bevacizumab in neoadjuvant therapy for locally advanced rectal cancer patients: A systematic review and meta-analysis
- PMID: 33248411
- PMCID: PMC7704460
- DOI: 10.1016/j.tranon.2020.100964
The efficacy and safety of adding bevacizumab in neoadjuvant therapy for locally advanced rectal cancer patients: A systematic review and meta-analysis
Abstract
Background: Patients with locally advanced rectal cancer (LARC) are more likely to suffer local recurrence and distant metastases, contributing to worse prognoses. Considering the provided dramatic reduction of local recurrences, neoadjuvant CRT (nCRT) followed by curative resection with total mesorectal excision (TME) and adjuvant chemotherapy has been established as standard therapy for LARC patients. However, the efficacy of adding bevacizumab in neoadjuvant therapy, especially in induction therapy-containing nCRT for LARC patients remains uncertain.
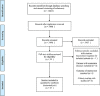
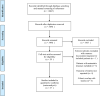
Materials: PubMed, Embase, and Web of Science were searched to retrieve records on the application of bevacizumab in a neoadjuvant setting for LARC patients. The endpoints of interest were pCR and the rates of patients suffering Grade 3/4 bevacizumab-specific adverse events, namely bleeding, wound healing complications, and gastrointestinal perforation.
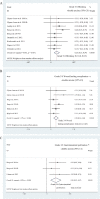
Results: 29 cohorts covering 1134 subjects were included in this systematic review. The pooled pCR rate for bevacizumab-relevant cohorts was 21% (95% confidence interval (95% CI), 17-25%; I2 = 61.8%), the pooled estimates of Grade 3/4 bleeding, Grade 3/4 wound healing complication, Grade 3/4 gastrointestinal perforation were 1% (95% CI, 0-3%; I2 = 0%), 2% (95% CI, 1-5%; I2 = 4.7%), and 2% (95% CI, 0-5%; I2 = 0%), respectively.
Conclusion: The addition of bevacizumab in the nCRT, especially in the TNT, for LARC patients provides promising efficacy and acceptable safety. However, the results should be interpreted cautiously due to the small amount of relevant data and need further confirmation by future studies.
Keywords: Bevacizumab; Induction therapy; Locally advanced rectal cancer; Neoadjuvant chemoradiotherapy; VEGF-inhibitor.
Copyright © 2020. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
The Addition of EGFR Inhibitors in Neoadjuvant Therapy for KRAS-Wild Type Locally Advanced Rectal Cancer Patients: A Systematic Review and Meta-Analysis.Front Pharmacol. 2020 May 15;11:706. doi: 10.3389/fphar.2020.00706. eCollection 2020. Front Pharmacol. 2020. PMID: 32499700 Free PMC article.
-
[Comparison of short-term efficacy and perioperative safety between neoadjuvant therapy and total neoadjuvant therapy in patients with locally advanced rectal cancer].Zhonghua Wei Chang Wai Ke Za Zhi. 2020 Mar 25;23(3):274-280. doi: 10.3760/cma.j.cn.441530-20190819-00312. Zhonghua Wei Chang Wai Ke Za Zhi. 2020. PMID: 32192307 Chinese.
-
Total neoadjuvant therapy versus standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis of 15 trials.PLoS One. 2022 Nov 4;17(11):e0276599. doi: 10.1371/journal.pone.0276599. eCollection 2022. PLoS One. 2022. PMID: 36331947 Free PMC article.
-
[Analysis on efficacy and safety of total neoadjuvant therapy in patients with locally advanced rectal cancer with high risk factors].Zhonghua Wei Chang Wai Ke Za Zhi. 2019 Apr 25;22(4):349-356. doi: 10.3760/cma.j.issn.1671-0274.2019.04.007. Zhonghua Wei Chang Wai Ke Za Zhi. 2019. PMID: 31054549 Chinese.
-
Efficacy and Safety of Two Neoadjuvant Strategies With Bevacizumab in MRI-Defined Locally Advanced T3 Resectable Rectal Cancer: Final Results of a Randomized, Noncomparative Phase 2 INOVA Study.Clin Colorectal Cancer. 2019 Sep;18(3):200-208.e1. doi: 10.1016/j.clcc.2019.04.006. Epub 2019 May 3. Clin Colorectal Cancer. 2019. PMID: 31311761 Clinical Trial.
Cited by
-
Enhancing clinical complete response assessment in rectal cancer: integrating transanal multipoint full-layer puncture biopsy criteria: a systematic review.Front Oncol. 2024 Dec 20;14:1428583. doi: 10.3389/fonc.2024.1428583. eCollection 2024. Front Oncol. 2024. PMID: 39759129 Free PMC article. Review.
-
Neoadjuvant Chemotherapy plus Bevacizumab Combined with Total Mesorectal Excision in Treating Locally Advanced Rectal Cancer Patients with BRAF Mutation: Clinical Benefit and Safety.Comput Math Methods Med. 2021 Dec 9;2021:4227650. doi: 10.1155/2021/4227650. eCollection 2021. Comput Math Methods Med. 2021. PMID: 34925539 Free PMC article.
-
Non-operative management after chemoradiotherapy plus consolidation or sandwich (induction with bevacizumab and consolidation) chemotherapy in patients with locally advanced rectal cancer: a multicentre, randomised phase II trial (NOMINATE trial).BMJ Open. 2022 Mar 18;12(3):e055140. doi: 10.1136/bmjopen-2021-055140. BMJ Open. 2022. PMID: 35304396 Free PMC article. Clinical Trial.
-
Can angiogenesis inhibitor therapy cause changes in imaging features of hepatic hemangioma- Initial study.Front Oncol. 2023 Mar 10;13:1134179. doi: 10.3389/fonc.2023.1134179. eCollection 2023. Front Oncol. 2023. PMID: 36969035 Free PMC article.
-
Adding Induction Chemotherapy Before Chemoradiotherapy with Total Mesorectal Excision and Selective Lateral Lymph Node Dissection for Patients with Poor-Risk, Locally Advanced, Mid-to-Low Rectal Cancer May Improve Oncologic Outcomes: A Propensity Score-Matched Analysis.Ann Surg Oncol. 2023 Aug;30(8):4716-4724. doi: 10.1245/s10434-023-13458-8. Epub 2023 Apr 9. Ann Surg Oncol. 2023. PMID: 37032405
References
-
- Siegel R.L., Miller K.D., Fedewa S.A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67(3):177e93. - PubMed
-
- Bosset J.F., Collette L., Calais G., Mineur L., Maingon P., Radosevic-Jelic L. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 2006;355:1114–1123. - PubMed
-
- Sauer R., Becker H., Hohenberger W., Rodel C., Wittekind C., Fietkau R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 2004;351:1731–1740. - PubMed
-
- Rectal cancer V.2+. 2019. NCCN Clinical Practical Guidelines in Oncology. Available at: http://www.nccn.org/professionals/physician_gls/.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

