Caenorhabditis elegans in anthelmintic research - Old model, new perspectives
- PMID: 33249235
- PMCID: PMC7704361
- DOI: 10.1016/j.ijpddr.2020.09.005
Caenorhabditis elegans in anthelmintic research - Old model, new perspectives
Abstract
For more than four decades, the free-living nematode Caenorhabditis elegans has been extensively used in anthelmintic research. Classic genetic screens and heterologous expression in the C. elegans model enormously contributed to the identification and characterization of molecular targets of all major anthelmintic drug classes. Although these findings provided substantial insights into common anthelmintic mechanisms, a breakthrough in the treatment and control of parasitic nematodes is still not in sight. Instead, we are facing increasing evidence that the enormous diversity within the phylum Nematoda cannot be recapitulated by any single free-living or parasitic species and the development of novel broad-spectrum anthelmintics is not be a simple goal. In the present review, we summarize certain milestones and challenges of the C. elegans model with focus on drug target identification, anthelmintic drug discovery and identification of resistance mechanisms. Furthermore, we present new perspectives and strategies on how current progress in C. elegans research will support future anthelmintic research.
Keywords: Anthelmintic drug; Anthelmintic resistance; Caenorhabditis elegans; Mode of action; Parasitic nematode.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Steffen R. Hahnel (SRH), Iring Heisler (IH) and Daniel Kulke (DK) are employees of Elanco Animal Health. Elanco Animal Health develops and sells veterinary pharmaceuticals including dewormers. Except of SRH, IH and DK, Elanco Animal Health was not involved in the preparation of the manuscript. The decision to publish the manuscript was jointly taken.
Figures
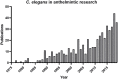
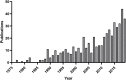

References
-
- Aboobaker A.A., Blaxter M.L. Use of RNA interference to investigate gene function in the human filarial nematode parasite Brugia malayi. Mol. Biochem. Parasitol. 2003;129:41–51. - PubMed
-
- Adlimoghaddam A., Weihrauch D., O'Donnell M.J. Localization of K(+), H(+), Na(+) and Ca(2)(+) fluxes to the excretory pore in Caenorhabditis elegans: application of scanning ion-selective microelectrodes. J. Exp. Biol. 2014;217:4119–4122. - PubMed
-
- Altreuther G., Buch J., Charles S.D., Davis W.L., Krieger K.J., Radeloff I. Field evaluation of the efficacy and safety of emodepside/praziquantel spot-on solution against naturally acquired nematode and cestode infections in domestic cats. Parasitol. Res. 2005;97(Suppl. 1):S58–s64. - PubMed
-
- Andersen E.C., Shimko T.C., Crissman J.R., Ghosh R., Bloom J.S., Seidel H.S., Gerke J.P., Kruglyak L. A powerful new quantitative genetics platform, combining Caenorhabditis elegans high-throughput fitness assays with a large collection of recombinant strains. G3 (Bethesda, Md.) 2015;5:911–920. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

