Multiple Endocrine Neoplasia Type 1: Latest Insights
- PMID: 33249439
- PMCID: PMC7958143
- DOI: 10.1210/endrev/bnaa031
Multiple Endocrine Neoplasia Type 1: Latest Insights
Abstract
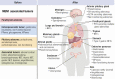
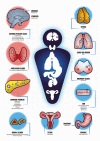
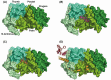
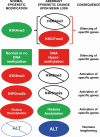
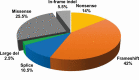
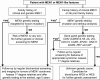
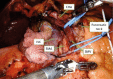
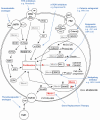
Multiple endocrine neoplasia type 1 (MEN1), a rare tumor syndrome that is inherited in an autosomal dominant pattern, is continuing to raise great interest for endocrinology, gastroenterology, surgery, radiology, genetics, and molecular biology specialists. There have been 2 major clinical practice guidance papers published in the past 2 decades, with the most recent published 8 years ago. Since then, several new insights on the basic biology and clinical features of MEN1 have appeared in the literature, and those data are discussed in this review. The genetic and molecular interactions of the MEN1-encoded protein menin with transcription factors and chromatin-modifying proteins in cell signaling pathways mediated by transforming growth factor β/bone morphogenetic protein, a few nuclear receptors, Wnt/β-catenin, and Hedgehog, and preclinical studies in mouse models have facilitated the understanding of the pathogenesis of MEN1-associated tumors and potential pharmacological interventions. The advancements in genetic diagnosis have offered a chance to recognize MEN1-related conditions in germline MEN1 mutation-negative patients. There is rapidly accumulating knowledge about clinical presentation in children, adolescents, and pregnancy that is translatable into the management of these very fragile patients. The discoveries about the genetic and molecular signatures of sporadic neuroendocrine tumors support the development of clinical trials with novel targeted therapies, along with advancements in diagnostic tools and surgical approaches. Finally, quality of life studies in patients affected by MEN1 and related conditions represent an effort necessary to develop a pharmacoeconomic interpretation of the problem. Because advances are being made both broadly and in focused areas, this timely review presents and discusses those studies collectively.
Keywords: MEN1; epigenetics; menin; mouse models; mutation-negative; neuroendocrine tumors; pharmacological therapies; phenocopy; quality of life; surgical approaches.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276(5311):404-407. - PubMed
-
- Lemmens I, Van de Ven WJ, Kas K, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet. 1997;6(7):1177-1183. - PubMed
-
- Thakker RV, Newey PJ, Walls GV, et al. ; Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97(9):2990-3011. - PubMed
-
- Giusti F, Cianferotti L, Boaretto F, et al. Multiple endocrine neoplasia syndrome type 1: institution, management, and data analysis of a nationwide multicenter patient database. Endocrine. 2017;58(2):349-359. - PubMed
-
- Carroll RW. Multiple endocrine neoplasia type 1 (MEN1). Asia Pac J Clin Oncol. 2013;9(4):297-309. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

