Migraine Headache Day Response Rates and the Implications to Patient Functioning: An Evaluation of 3 Randomized Phase 3 Clinical Trials of Galcanezumab in Patients With Migraine
- PMID: 33249580
- PMCID: PMC7756324
- DOI: 10.1111/head.14013
Migraine Headache Day Response Rates and the Implications to Patient Functioning: An Evaluation of 3 Randomized Phase 3 Clinical Trials of Galcanezumab in Patients With Migraine
Erratum in
-
Erratum.Headache. 2021 Jun;61(6):977. doi: 10.1111/head.14079. Epub 2021 Jan 31. Headache. 2021. PMID: 34214184 Free PMC article. No abstract available.
Abstract
Objective: This post hoc study investigated the relationship between patient response in terms of migraine headache day reduction and patient-reported outcomes of health-related quality of life (HRQoL) and disability categories.
Background: Migraine causes considerable disease-related disability and negatively impacts HRQoL of patients. Calcitonin gene-related peptide inhibitors improve these outcomes and may eliminate disability due to migraine in some patients.
Methods: Analyses used data from 3 double-blind, placebo (PBO)-controlled, phase 3 studies in adults with episodic migraine (EM) (EVOLVE-1: N = 858 and EVOLVE-2: N = 915) or chronic migraine (CM) (REGAIN: N = 1113). Patients were randomized 2:1:1 to subcutaneous injection of PBO, galcanezumab (GMB) 120 mg, or GMB 240 mg once monthly for 6 months in EVOLVE-1 and -2 and for 3 months in REGAIN. Primary endpoint was overall mean change from baseline in monthly migraine headache days. Patients were divided into 4 response-level groups based on percent change from baseline (<30%, ≥30% to <50%, ≥50% to <75%, ≥75%). Patient-reported outcomes included the 14-item Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ) and Migraine Disability Assessment (MIDAS) questionnaire.
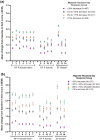
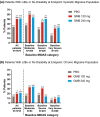
Results: Among patients with migraine, mean improvements from baseline in MSQ domain scores increased with each successive level of migraine headache day response. On a 100-pt scale, increases in Role Function-Restrictive score in EM were 16.8 and 36.0 at the <30% and ≥75% response levels, respectively, and for CM were 10.7 and 46.5. Similar patterns in scores were observed for the Role Function-Preventive and Emotional Function domains. Examination of improvement in MSQ item score by treatment group showed that, in patients with EM, approximately 10 to 20% more GMB-treated patients (N = 796 for GMB 120 mg and GMB 240 mg) had improvements in all 14 MSQ items compared with PBO-treated patients (N = 773) (all P < .001). In patients with CM, 3 to 16% more GMB-treated patients (N = 507) had improvements in the 14 MSQ items compared with PBO (N = 494), though differences were statistically significant in only 19 of 28 comparisons. At baseline, mean MIDAS scores (EM, 33.1; CM, 67.2) indicated severe mean disability for patients with EM and very severe disability for patients with CM. Among patients with EM, 215 of 425 (50.6%) of those treated with GMB 120 mg and 212 of 413 (51.3%) treated with 240 mg had little/no disability due to migraine after 6 months (PBO: 277 of 832 (33.3%), P < .001 for both). Among patients with CM, 50 of 254 (19.7%) of those treated with GMB 120 mg and 54 of 258 (20.9%) treated with 240 mg reached the level of little/no disability after 3 months of treatment (PBO: 70 of 504 (13.9%), P = .045 for 120 mg, P = .017 for 240 mg).
Conclusions: Because migraine greatly impairs an individual's ability to participate in activities of daily living, measurements of HRQoL are essential in clinical research. This study showed that function in daily life, as measured by MSQ score, improved as migraine headache days were reduced and that GMB-treated patients were more likely to see improvement in MSQ item scores compared with PBO-treated patients. Elimination of migraine-related disability was also more frequent in GMB-treated patients compared with placebo-treated patients.
Keywords: calcitonin gene-related peptide; chronic migraine; episodic migraine; galcanezumab; patient-reported outcomes.
© 2020 Eli Lilly and Company. Headache: The Journal of Head and Face Pain published by Wiley Periodicals LLC, on behalf of American Headache Society.
Figures
Similar articles
-
Annual indirect costs savings in patients with episodic or chronic migraine: a post-hoc analysis of phase 3 galcanezumab clinical trials in the United States.J Med Econ. 2023 Jan-Dec;26(1):149-157. doi: 10.1080/13696998.2023.2165365. J Med Econ. 2023. PMID: 36601798 Clinical Trial.
-
Functional impairment and disability among patients with migraine: evaluation of galcanezumab in a long-term, open-label study.Qual Life Res. 2021 Feb;30(2):455-464. doi: 10.1007/s11136-020-02632-0. Epub 2020 Sep 17. Qual Life Res. 2021. PMID: 32944843 Free PMC article. Clinical Trial.
-
Migraine-Specific Quality-of-Life Questionnaire (MSQ) Version 2.1 Score Improvement in Japanese Patients with Episodic Migraine by Galcanezumab Treatment: Japan Phase 2 Study.J Pain Res. 2020 Dec 31;13:3531-3538. doi: 10.2147/JPR.S287781. eCollection 2020. J Pain Res. 2020. PMID: 33408512 Free PMC article. Clinical Trial.
-
Efficacy and Safety of Galcanezumab for the Preventive Treatment of Migraine: A Narrative Review.Adv Ther. 2020 May;37(5):2034-2049. doi: 10.1007/s12325-020-01319-9. Epub 2020 Apr 21. Adv Ther. 2020. PMID: 32319039 Free PMC article. Review.
-
Onset, Maintenance, and Cessation of Effect of Galcanezumab for Prevention of Migraine: A Narrative Review of Three Randomized Placebo-Controlled Trials.Adv Ther. 2021 Mar;38(3):1614-1626. doi: 10.1007/s12325-021-01632-x. Epub 2021 Feb 5. Adv Ther. 2021. PMID: 33544305 Free PMC article. Review.
Cited by
-
Erenumab versus topiramate: migraine-related disability, impact and health-related quality of life.Eur J Neurol. 2024 Dec;31(12):e16437. doi: 10.1111/ene.16437. Epub 2024 Aug 12. Eur J Neurol. 2024. PMID: 39132915 Free PMC article. Clinical Trial.
-
Galcanezumab effects on incidence of headache after occurrence of triggers, premonitory symptoms, and aura in responders, non-responders, super-responders, and super non-responders.J Headache Pain. 2023 Mar 16;24(1):26. doi: 10.1186/s10194-023-01560-x. J Headache Pain. 2023. PMID: 36927366 Free PMC article.
-
In search of a gold standard patient-reported outcome measure to use in the evaluation and treatment-decision making in migraine prevention. A real-world evidence study.J Headache Pain. 2021 Dec 13;22(1):151. doi: 10.1186/s10194-021-01366-9. J Headache Pain. 2021. PMID: 34903177 Free PMC article.
-
Erratum.Headache. 2021 Jun;61(6):977. doi: 10.1111/head.14079. Epub 2021 Jan 31. Headache. 2021. PMID: 34214184 Free PMC article. No abstract available.
-
Rates of Response to Atogepant for Migraine Prophylaxis Among Adults: A Secondary Analysis of a Randomized Clinical Trial.JAMA Netw Open. 2022 Jun 1;5(6):e2215499. doi: 10.1001/jamanetworkopen.2022.15499. JAMA Netw Open. 2022. PMID: 35675076 Free PMC article. Clinical Trial.
References
-
- Freitag FG. The cycle of migraine: Patients' quality of life during and between migraine attacks. Clin Ther. 2007;29:939‐949. - PubMed
-
- Bigal ME, Rapoport AM, Lipton RB, Tepper SJ, Sheftell FD. Assessment of migraine disability using the migraine disability assessment (MIDAS) questionnaire: A comparison of chronic migraine with episodic migraine. Headache. 2003;43:336‐342. - PubMed
-
- Guitera V, Munoz P, Castillo J, Pascual J. Quality of life in chronic daily headache: A study in a general population. Neurology. 2002;58:1062‐1065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

