Impact of Genetic and Pharmacologic Inhibition of Myostatin in a Murine Model of Osteogenesis Imperfecta
- PMID: 33249643
- PMCID: PMC8111798
- DOI: 10.1002/jbmr.4223
Impact of Genetic and Pharmacologic Inhibition of Myostatin in a Murine Model of Osteogenesis Imperfecta
Abstract
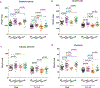
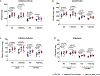
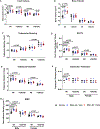
Osteogenesis imperfecta (OI) is a genetic connective tissue disorder characterized by compromised skeletal integrity, altered microarchitecture, and bone fragility. Current OI treatment strategies focus on bone antiresorptives and surgical intervention with limited effectiveness, and thus identifying alternative therapeutic options remains critical. Muscle is an important stimulus for bone formation. Myostatin, a TGF-β superfamily myokine, acts through ActRIIB to negatively regulate muscle growth. Recent studies demonstrated the potential benefit of myostatin inhibition with the soluble ActRIIB fusion protein on skeletal properties, although various OI mouse models exhibited variable skeletal responses. The genetic and clinical heterogeneity associated with OI, the lack of specificity of the ActRIIB decoy molecule for myostatin alone, and adverse events in human clinical trials further the need to clarify myostatin's therapeutic potential and role in skeletal integrity. In this study, we determined musculoskeletal outcomes of genetic myostatin deficiency and postnatal pharmacological myostatin inhibition by a monoclonal anti-myostatin antibody (Regn647) in the G610C mouse, a model of mild-moderate type I/IV human OI. In the postnatal study, 5-week-old wild-type and +/G610C male and female littermates were treated with Regn647 or a control antibody for 11 weeks or for 7 weeks followed by a 4-week treatment holiday. Inhibition of myostatin, whether genetically or pharmacologically, increased muscle mass regardless of OI genotype, although to varying degrees. Genetic myostatin deficiency increased hindlimb muscle weights by 6.9% to 34.4%, whereas pharmacological inhibition increased them by 13.5% to 29.6%. Female +/mstn +/G610C (Dbl.Het) mice tended to have similar trabecular and cortical bone parameters as Wt showing reversal of +/G610C characteristics but with minimal effect of +/mstn occurring in male mice. Pharmacologic myostatin inhibition failed to improve skeletal bone properties of male or female +/G610C mice, although skeletal microarchitectural and biomechanical improvements were observed in male wild-type mice. Four-week treatment holiday did not alter skeletal outcomes. © 2020 American Society for Bone and Mineral Research (ASBMR).
Keywords: BONE-MUSCLE INTERACTIONS; COL1A2; OSTEOGENESIS IMPERFECTA (OI); PRECLINICAL STUDIES; TGF-Β.
© 2020 American Society for Bone and Mineral Research (ASBMR).
Conflict of interest statement
Disclosures
SK provided the Regn647 and Regn1945 (Regeneron Pharmaceuticals Inc., Tarrytown, NY, USA), is an employee of Regeneron Pharmaceuticals, and owns stock of this company. LM, JM, and AR are employees of Regeneron Pharmaceuticals. All other authors state that they have no conflicts of interest.
Figures








References
-
- Folkestad L, Hald JD, Hansen S, et al. Bone geometry, density, and microarchitecture in the distal radius and tibia in adults with osteogenesis imperfecta type I assessed by high-resolution pQCT. J Bone Miner Res. 2012;27(6):1405–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

