Interleukin 6 trans-signaling is a critical driver of lung allograft fibrosis
- PMID: 33249747
- PMCID: PMC8809084
- DOI: 10.1111/ajt.16417
Interleukin 6 trans-signaling is a critical driver of lung allograft fibrosis
Abstract
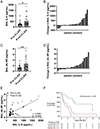
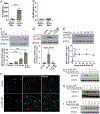
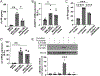
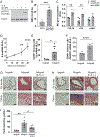
Histopathologic examination of lungs afflicted by chronic lung allograft dysfunction (CLAD) consistently shows both mononuclear cell (MNC) inflammation and mesenchymal cell (MC) fibroproliferation. We hypothesize that interleukin 6 (IL-6) trans-signaling may be a critical mediator of MNC-MC crosstalk and necessary for the pathogenesis of CLAD. Bronchoalveolar lavage (BAL) fluid obtained after the diagnosis of CLAD has approximately twofold higher IL-6 and soluble IL-6 receptor (sIL-6R) levels compared to matched pre-CLAD samples. Human BAL-derived MCs do not respond to treatment with IL-6 alone but have rapid and prolonged JAK2-mediated STAT3 Tyr705 phosphorylation when exposed to the combination of IL-6 and sIL-6R. STAT3 phosphorylation within MCs upregulates numerous genes causing increased invasion and fibrotic differentiation. MNC, a key source of both IL-6 and sIL-6R, produce minimal amounts of these proteins at baseline but significantly upregulate production when cocultured with MCs. Finally, the use of an IL-6 deficient recipient in a murine orthotopic transplant model of CLAD reduces allograft fibrosis by over 50%. Taken together these results support a mechanism where infiltrating MNCs are stimulated by resident MCs to release large quantities of IL-6 and sIL-6R which then feedback onto the MCs to increase invasion and fibrotic differentiation.
Keywords: animal models: murine; basic (laboratory) research / science; bronchiolitis obliterans (BOS); cellular biology; cytokines / cytokine receptors; fibrosis; lung transplantation / pulmonology; translational research / science.
© 2020 The American Society of Transplantation and the American Society of Transplant Surgeons.
Conflict of interest statement
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the
Figures

References
-
- Verleden GM, Glanville AR, Lease ED, Fisher AJ, Calabrese F, Corris PA et al. Chronic lung allograft dysfunction: Definition, diagnostic criteria, and approaches to treatment-A consensus report from the Pulmonary Council of the ISHLT. J Heart Lung Transplant 2019;38(5):493–503. - PubMed
-
- von der Thusen JH, Vandermeulen E, Vos R, Weynand B, Verbeken EK, Verleden SE. The histomorphological spectrum of restrictive chronic lung allograft dysfunction and implications for prognosis. Mod Pathol 2018;31(5):780–790. - PubMed
-
- Martinu T, Howell DN, Davis RD, Steele MP, Palmer SM. Pathologic correlates of bronchiolitis obliterans syndrome in pulmonary retransplant recipients. Chest 2006;129(4):1016–1023. - PubMed
-
- Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant 1998;17(12):1255–1263. - PubMed
-
- Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant 2002;21(2):271–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

