Inhibitory evaluation of Curculigo latifolia on α-glucosidase, DPP (IV) and in vitro studies in antidiabetic with molecular docking relevance to type 2 diabetes mellitus
- PMID: 33249946
- PMCID: PMC7717572
- DOI: 10.1080/14756366.2020.1844680
Inhibitory evaluation of Curculigo latifolia on α-glucosidase, DPP (IV) and in vitro studies in antidiabetic with molecular docking relevance to type 2 diabetes mellitus
Abstract
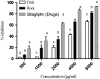
The inhibition of α-glucosidase and DPP enzymes capable of effectively reducing blood glucose level in the management of type 2 diabetes. The purpose of the present study is to evaluate the inhibitory potential of α-glucosidase and DPP (IV) activity including with the 2-NBDG uptake assay and insulin secretion activities through in vitro studies. The selected of active compounds obtained from the screening of compounds by LC-MS were docked with the targeted enzyme that involved in the mechanism of T2DM. From the results, root extracts displayed a better promising outcome in α-glucosidase (IC50 2.72 ± 0.32) as compared with the fruit extracts (IC50 3.87 ± 0.32). Besides, root extracts also displayed a better activity in the inhibition of DPP (IV), enhance insulin secretion and glucose uptake activity. Molecular docking results revealing that phlorizin binds strongly with α-glucosidase, DPP (IV) and Insulin receptor (IR) enzymes with achieving the lowest binding energy value. The present work suggests several of the compounds have the potential that contribute towards inhibiting α-glucosidase and DPP (IV) and thus effective in lowering post-prandial hyperglycaemia.
Keywords: Curculigo latifolia; DPP (IV); molecular docking; type 2 diabetes; α-glucosidase.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures







References
-
- Okur ME, Karantas ID, Siafaka PI.. Diabetes mellitus: a review on pathophysiology, current status of oral pathophysiology, current status of oral medications and future perspectives. ACTA Pharmaceutica Sciencia 2017;55:61.
-
- Cho N, Shaw JE, Karuranga S, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabet Res Clin Pract 2018;138:271–81. - PubMed
-
- Yuen L, Saeedi P, Riaz M, et al. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract 2019;157:107841. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
