Modeling the Interactions Between Sodium Channels Provides Insight Into the Negative Dominance of Certain Channel Mutations
- PMID: 33250780
- PMCID: PMC7674773
- DOI: 10.3389/fphys.2020.589386
Modeling the Interactions Between Sodium Channels Provides Insight Into the Negative Dominance of Certain Channel Mutations
Abstract
Background: Nav1.5 cardiac Na+ channel mutations can cause arrhythmogenic syndromes. Some of these mutations exert a dominant negative effect on wild-type channels. Recent studies showed that Na+ channels can dimerize, allowing coupled gating. This leads to the hypothesis that allosteric interactions between Na+ channels modulate their function and that these interactions may contribute to the negative dominance of certain mutations.
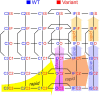
Methods: To investigate how allosteric interactions affect microscopic and macroscopic channel function, we developed a modeling paradigm in which Markovian models of two channels are combined. Allosteric interactions are incorporated by modifying the free energies of the composite states and/or barriers between states.
Results: Simulations using two generic 2-state models (C-O, closed-open) revealed that increasing the free energy of the composite states CO/OC leads to coupled gating. Simulations using two 3-state models (closed-open-inactivated) revealed that coupled closings must also involve interactions between further composite states. Using two 6-state cardiac Na+ channel models, we replicated previous experimental results mainly by increasing the energies of the CO/OC states and lowering the energy barriers between the CO/OC and the CO/OO states. The channel model was then modified to simulate a negative dominant mutation (Nav1.5 p.L325R). Simulations of homodimers and heterodimers in the presence and absence of interactions showed that the interactions with the variant channel impair the opening of the wild-type channel and thus contribute to negative dominance.
Conclusion: Our new modeling framework recapitulates qualitatively previous experimental observations and helps identifying possible interaction mechanisms between ion channels.
Keywords: Markov models; allosteric interactions; cardiac electrophysiology; computer modeling; sodium channels; sodium current; statistical mechanics.
Copyright © 2020 Hichri, Selimi and Kucera.
Figures










Similar articles
-
Mutant voltage-gated Na+ channels can exert a dominant negative effect through coupled gating.Am J Physiol Heart Circ Physiol. 2018 Nov 1;315(5):H1250-H1257. doi: 10.1152/ajpheart.00721.2017. Epub 2018 Aug 17. Am J Physiol Heart Circ Physiol. 2018. PMID: 30118344 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Modeling of single noninactivating Na+ channels: evidence for two open and several fast inactivated states.Biophys J. 2006 May 15;90(10):3511-22. doi: 10.1529/biophysj.105.073072. Epub 2006 Mar 2. Biophys J. 2006. PMID: 16513781 Free PMC article.
-
Trafficking and Gating Cooperation Between Deficient Nav1.5-mutant Channels to Rescue INa.Front Biosci (Landmark Ed). 2022 Jun 30;27(7):209. doi: 10.31083/j.fbl2707209. Front Biosci (Landmark Ed). 2022. PMID: 35866404
-
An integrated view of the molecular toxinology of sodium channel gating in excitable cells.Annu Rev Neurosci. 1987;10:237-67. doi: 10.1146/annurev.ne.10.030187.001321. Annu Rev Neurosci. 1987. PMID: 2436544 Review.
Cited by
-
Mechanisms and physiological implications of cooperative gating of clustered ion channels.Physiol Rev. 2022 Jul 1;102(3):1159-1210. doi: 10.1152/physrev.00022.2021. Epub 2021 Dec 20. Physiol Rev. 2022. PMID: 34927454 Free PMC article. Review.
-
Variability in reported midpoints of (in)activation of cardiac INa.J Gen Physiol. 2025 Sep 1;157(5):e202413621. doi: 10.1085/jgp.202413621. Epub 2025 Jul 16. J Gen Physiol. 2025. PMID: 40668222 Free PMC article. Review.
-
BK channels of five different subunit combinations underlie the de novo KCNMA1 G375R channelopathy.J Gen Physiol. 2023 May 1;155(5):e202213302. doi: 10.1085/jgp.202213302. Epub 2023 Mar 30. J Gen Physiol. 2023. PMID: 36995317 Free PMC article.
References
-
- Barnett M. W., Larkman P. M. (2007). The action potential. Pract. Neurol. 7 192–197. - PubMed
-
- Bondarenko V. E., Szigeti G. P., Bett G. C., Kim S. J., Rasmusson R. L. (2004). Computer model of action potential of mouse ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 287 H1378–H1403. - PubMed

