Iron Uptake Mechanisms in Marine Phytoplankton
- PMID: 33250865
- PMCID: PMC7676907
- DOI: 10.3389/fmicb.2020.566691
Iron Uptake Mechanisms in Marine Phytoplankton
Abstract
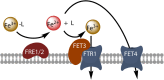
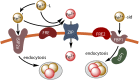
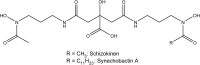
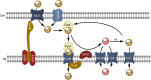
Oceanic phytoplankton species have highly efficient mechanisms of iron acquisition, as they can take up iron from environments in which it is present at subnanomolar concentrations. In eukaryotes, three main models were proposed for iron transport into the cells by first studying the kinetics of iron uptake in different algal species and then, more recently, by using modern biological techniques on the model diatom Phaeodactylum tricornutum. In the first model, the rate of uptake is dependent on the concentration of unchelated Fe species, and is thus limited thermodynamically. Iron is transported by endocytosis after carbonate-dependent binding of Fe(III)' (inorganic soluble ferric species) to phytotransferrin at the cell surface. In this strategy the cells are able to take up iron from very low iron concentration. In an alternative model, kinetically limited for iron acquisition, the extracellular reduction of all iron species (including Fe') is a prerequisite for iron acquisition. This strategy allows the cells to take up iron from a great variety of ferric species. In a third model, hydroxamate siderophores can be transported by endocytosis (dependent on ISIP1) after binding to the FBP1 protein, and iron is released from the siderophores by FRE2-dependent reduction. In prokaryotes, one mechanism of iron uptake is based on the use of siderophores excreted by the cells. Iron-loaded siderophores are transported across the cell outer membrane via a TonB-dependent transporter (TBDT), and are then transported into the cells by an ABC transporter. Open ocean cyanobacteria do not excrete siderophores but can probably use siderophores produced by other organisms. In an alternative model, inorganic ferric species are transported through the outer membrane by TBDT or by porins, and are taken up by the ABC transporter system FutABC. Alternatively, ferric iron of the periplasmic space can be reduced by the alternative respiratory terminal oxidase (ARTO) and the ferrous ions can be transported by divalent metal transporters (FeoB or ZIP). After reoxidation, iron can be taken up by the high-affinity permease Ftr1.
Keywords: iron; iron uptake; micro-algae; ocean; phytoplankton.
Copyright © 2020 Sutak, Camadro and Lesuisse.
Figures

References
-
- Allen M. D., Del Campo J. A., Kropat J., Merchant S. S. (2007). FEA1, FEA2, and FRE1, encoding two homologous secreted proteins and a candidate ferrireductase, are expressed coordinately with FOX1 and FTR1 in iron-deficient Chlamydomonas reinhardtii. Eukaryot. Cell 6 1841–1852. 10.1128/EC.00205-07 - DOI - PMC - PubMed
-
- Anderson M. A., Morel F. M. M. (1982). The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol. Oceanogr. 27 789–813. 10.4319/lo.1982.27.5.0789 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

