C=C-Ene-Reductases Reduce the C=N Bond of Oximes
- PMID: 33251037
- PMCID: PMC7685226
- DOI: 10.1021/acscatal.0c03755
C=C-Ene-Reductases Reduce the C=N Bond of Oximes
Abstract
Although enzymes have been found for many reactions, there are still transformations for which no enzyme is known. For instance, not a single defined enzyme has been described for the reduction of the C=N bond of an oxime, only whole organisms. Such an enzymatic reduction of an oxime may give access to (chiral) amines. By serendipity, we found that the oxime moiety adjacent to a ketone as well as an ester group can be reduced by ene-reductases (ERs) to an intermediate amino group. ERs are well-known enzymes for the reduction of activated alkenes, as of α,β-unsaturated ketones. For the specific substrate used here, the amine intermediate spontaneously reacts further to tetrasubstituted pyrazines. This reduction reaction represents an unexpected promiscuous activity of ERs expanding the toolkit of transformations using enzymes.
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



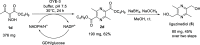
Similar articles
-
Mechanistic Insights into the Ene-Reductase-Catalyzed Promiscuous Reduction of Oximes to Amines.ACS Catal. 2023 Feb 6;13(4):2610-2618. doi: 10.1021/acscatal.2c06137. eCollection 2023 Feb 17. ACS Catal. 2023. PMID: 36846821 Free PMC article.
-
Metagenomic ene-reductases for the bioreduction of sterically challenging enones.RSC Adv. 2019 Nov 11;9(63):36608-36614. doi: 10.1039/c9ra06088j. eCollection 2019 Nov 11. RSC Adv. 2019. PMID: 35539044 Free PMC article.
-
Asymmetric Reductive Carbocyclization Using Engineered Ene Reductases.Angew Chem Int Ed Engl. 2018 Jun 11;57(24):7240-7244. doi: 10.1002/anie.201802962. Epub 2018 May 14. Angew Chem Int Ed Engl. 2018. PMID: 29689601 Free PMC article.
-
The azomethine ylide route to amine C-H functionalization: redox-versions of classic reactions and a pathway to new transformations.Acc Chem Res. 2015 Feb 17;48(2):317-28. doi: 10.1021/ar5003768. Epub 2015 Jan 6. Acc Chem Res. 2015. PMID: 25560649 Free PMC article. Review.
-
Ene-Reductase: A Multifaceted Biocatalyst in Organic Synthesis.Chemistry. 2022 Apr 12;28(21):e202103949. doi: 10.1002/chem.202103949. Epub 2022 Mar 3. Chemistry. 2022. PMID: 35133702 Review.
Cited by
-
Solvent concentration at 50% protein unfolding may reform enzyme stability ranking and process window identification.Nat Commun. 2024 Jun 26;15(1):5420. doi: 10.1038/s41467-024-49774-0. Nat Commun. 2024. PMID: 38926341 Free PMC article.
-
Catalytic Reduction of Oximes to Hydroxylamines: Current Methods, Challenges and Opportunities.Chemistry. 2022 Feb 21;28(10):e202103683. doi: 10.1002/chem.202103683. Epub 2021 Dec 22. Chemistry. 2022. PMID: 34817089 Free PMC article. Review.
-
Enzymatic strategies for asymmetric synthesis.RSC Chem Biol. 2021 Jun 1;2(4):958-989. doi: 10.1039/d1cb00080b. eCollection 2021 Aug 5. RSC Chem Biol. 2021. PMID: 34458820 Free PMC article. Review.
-
Unlocking the function promiscuity of old yellow enzyme to catalyze asymmetric Morita-Baylis-Hillman reaction.Nat Commun. 2024 Jul 9;15(1):5737. doi: 10.1038/s41467-024-50141-2. Nat Commun. 2024. PMID: 38982157 Free PMC article.
-
Imine reduction by an Ornithine cyclodeaminase/μ-crystallin homolog purified from Candida parapsilosis ATCC 7330.Biotechnol Rep (Amst). 2021 Aug 5;31:e00664. doi: 10.1016/j.btre.2021.e00664. eCollection 2021 Sep. Biotechnol Rep (Amst). 2021. PMID: 34557391 Free PMC article.
References
-
- Troshin K.; Hartwig J. F. Snap deconvolution: An informatics approach to high-throughput discovery of catalytic reactions. Science 2017, 357, 175–181. 10.1126/science.aan1568. - DOI - PubMed
- Collins K. D.; Gensch T.; Glorius F. Contemporary screening approaches to reaction discovery and development. Nat. Chem. 2014, 6, 859–871. 10.1038/nchem.2062. - DOI - PubMed
- Montgomery J. High-Throughput discovery of new chemical reactions. Science 2011, 333, 1387–1388. 10.1126/science.1210735. - DOI - PubMed
-
- Biegasiewicz K. F.; Cooper S. J.; Gao X.; Oblinsky D. G.; Kim J. H.; Garfinkle S. E.; Joyce L. A.; Sandoval B. A.; Scholes G. D.; Hyster T. K. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 2019, 364, 1166–1169. 10.1126/science.aaw1143. - DOI - PMC - PubMed
- Emmanuel M. A.; Greenberg N. R.; Oblinsky D. G.; Hyster T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 2016, 540, 414–417. 10.1038/nature20569. - DOI - PubMed
- Huang X.; Wang B.; Wang Y.; Jiang G.; Feng J.; Zhao H. Photoenzymatic enantioselective intermolecular radical hydroalkylation. Nature 2020, 584, 69–74. 10.1038/s41586-020-2406-6. - DOI - PubMed
-
- Zhang J.; Huang X.; Zhang R. K.; Arnold F. H. Enantiodivergent α-amino C-H fluoroalkylation catalyzed by engineered cytochrome P450s. J. Am. Chem. Soc. 2019, 141, 9798–9802. 10.1021/jacs.9b04344. - DOI - PMC - PubMed
- Zhang R. K.; Chen K.; Huang X.; Wohlschlager L.; Renata H.; Arnold F. H. Enzymatic assembly of carbon-carbon bonds via iron-catalysed sp3 C-H functionalization. Nature 2019, 565, 67–72. 10.1038/s41586-018-0808-5. - DOI - PMC - PubMed
- Chen K.; Huang X.; Kan S. B. J.; Zhang R. K.; Arnold F. H. Enzymatic construction of highly strained carbocycles. Science 2018, 360, 71–75. 10.1126/science.aar4239. - DOI - PMC - PubMed
- Jeschek M.; Reuter R.; Heinisch T.; Trindler C.; Klehr J.; Panke S.; Ward T. R. Directed evolution of artificial metalloenzymes for in vivo metathesis. Nature 2016, 537, 661–665. 10.1038/nature19114. - DOI - PubMed
-
- Li J.; Hu Y.; Zhang D.; Liu Q.; Dong Y.; Liu H. Transition Metal-Catalyzed Reactions Involving Oximes. Adv. Synth. Catal. 2017, 359, 710–771. 10.1002/adsc.201600807. - DOI
- Huang H.; Cai J.; Deng G.-J. O-Acyl oximes: versatile building blocks for N-heterocycle formation in recent transition metal catalysis. Org. Biomol. Chem. 2016, 14, 1519–1530. 10.1039/c5ob02417j. - DOI - PubMed
- Sukhorukov A. Y.; Ioffe S. L. Chemistry of six-membered cyclic oxime ethers. Application in the synthesis of bioactive compounds. Chem. Rev. 2011, 111, 5004–5041. 10.1021/cr100292w. - DOI - PubMed
-
- Crochet P.; Cadierno V. Catalytic synthesis of amides via aldoximes rearrangement. Chem. Commun. 2015, 51, 2495–2505. 10.1039/c4cc08684h. - DOI - PubMed
- Gawley R. E. Beckmann reactions: rearrangements, elimination-additions, fragmentations, and rearrangement-cyclizations. Org. React. 1988, 35, 1–420. 10.1002/0471264180.or035.01. - DOI
LinkOut - more resources
Full Text Sources
