Pathway engineering of Escherichia coli for one-step fermentative production of L-theanine from sugars and ethylamine
- PMID: 33251110
- PMCID: PMC7677707
- DOI: 10.1016/j.mec.2020.e00151
Pathway engineering of Escherichia coli for one-step fermentative production of L-theanine from sugars and ethylamine
Abstract
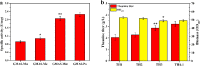
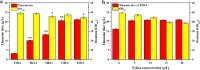
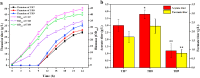
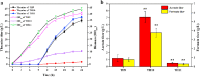
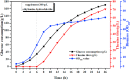
L-theanine is the most abundant free amino acid in tea that offers various favorable physiological and pharmacological effects. Bacterial enzyme of γ-glutamylmethylamide synthetase (GMAS) can catalyze the synthesis of theanine from glutamate, ethylamine and ATP, but the manufacturing cost is uncompetitive due to the expensive substrates and complex processes. In this study, we described pathway engineering of wild-type Escherichia coli for one-step fermentative production of theanine from sugars and ethylamine. First, the synthetic pathway of theanine was conducted by heterologous introduction of a novel GMAS from Paracoccus aminovorans. A xylose-induced T7 RNA polymerase-P T7 promoter system was used to enhance and control gmas gene expression. Next, the precursor glutamate pool was increased by overexpression of native citrate synthase and introduction of glutamate dehydrogenase from Corynebacterium glutamicum. Then, in order to push more carbon flux towards theanine synthesis, the tricarboxylic acid cycle was interrupted and pyruvate carboxylase from C. glutamicum was introduced as a bypath supplying oxaloacetate from pyruvate. Finally, an energy-conserving phosphoenolpyruvate carboxykinase from Mannheimia succiniciproducens was introduced to increase ATP yield for theanine synthesis. After optimizing the addition time and concentration of ethylamine hydrochloride in the fed-batch fermentation, the recombinant strain TH11 produced 70.6 g/L theanine in a 5-L bioreactor with a yield and productivity of 0.42 g/g glucose and 2.72 g/L/h, respectively. To our knowledge, this is the first report regarding the pathway engineering of E. coli for fermentative production of theanine. The high production capacity of recombinant strain, combined with the easy processes, will hold attractive industrial application potential for the future.
Keywords: Escherichia coli; Fermentative production; L-theanine; Pathway engineering; γ-Glutamylmethylamide synthetase.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

