Prospective Evaluation of All-lesion Versus Single-lesion Radiotherapy in Combination With PD-1/PD-L1 Immune Checkpoint Inhibitors
- PMID: 33251140
- PMCID: PMC7673414
- DOI: 10.3389/fonc.2020.576643
Prospective Evaluation of All-lesion Versus Single-lesion Radiotherapy in Combination With PD-1/PD-L1 Immune Checkpoint Inhibitors
Abstract
Background: Local ablative treatments improve survival in patients with oligometastatic disease in addition to chemotherapy. The application of immune checkpoint inhibitors prolonged patients' survival in different tumor entities. This raises the question if patients still benefit from intensified local treatments in combination with a more efficient systemic treatment with immune checkpoint inhibitors.
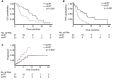
Methods: The prospective non-interventional ST-ICI trial investigates treatment with PD-1/PD-L1 (Programmed cell death protein 1/Programmed cell death 1 ligand 1) immune checkpoint inhibitors and radiotherapy in different tumor entities. Patients who started radiotherapy and immunotherapy concomitantly were included in this interim analysis. In this cohort patients with all-lesion radiotherapy (all tumor lesions irradiated, al-RT) were compared to patients with radiotherapy to only a single of their tumor lesions (single-lesion radiotherapy, sl-RT). Endpoints of the interim analysis were progression-free survival (PFS), overall survival (OS) and time to progression (TTP).
Results: A total of 104 patients were registered between April 2017 and August 2019. Fifty patients started immune checkpoint inhibitor treatment and radiotherapy concomitantly and were included. Most frequent tumor entities were non-small cell lung cancer (62%) followed by head and neck squamous cell cancer (26%). Most frequent location of radiotherapy was lung (34%) and central nervous system (20%). Median duration of follow-up was 8.6 months beginning with first administration of the immune-checkpoint-inhibitor. Median PFS was 9.2 months (95% CI, 5.8 - 12.6) in the al-RT group and 3.0 months (95% CI, 2.5 - 3.5) in the sl-RT group (p<0.001). Median OS was 11.6 months (95% CI, 8.1 - 15.1) in the al-RT group and 4.2 months (95% CI, 3.0 - 5.4) in the sl-RT group (p=0.007). Median TTP was not reached in the al-RT group compared to 4.6 months (95% CI, 1.1-8.0) in the sl-RT group (p=0.028). Univariate Cox regression analyses computed tumor entity, histology, central nervous system metastases, immunotherapy drug and al-RT as predictors of OS (with an effect p-value of ≤ 0.1). In the multivariable analysis only tumor entity and al-RT remained prognostic factors for OS.
Conclusion: Patients with PD-1/PD-L1 immune checkpoint inhibitor therapy benefit from local radiotherapy to all known lesions compared to single-lesion radiotherapy regarding PFS and OS.
Keywords: all-lesion; immune checkpoint inhibitors; immunotherapy; oligometastatic; radiotherapy; single-lesion.
Copyright © 2020 Schubert, Rutzner, Eckstein, Frey, Schweizer, Haderlein, Lettmaier, Semrau, Gostian, Zhou, Gaipl, Fietkau and Hecht.
Figures
References
-
- Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol (2020) 21(1):e18–28. 10.1016/S1470-2045(19)30718-1 - DOI - PubMed
-
- Gomez DR, Tang C, Zhang J, Blumenschein GR, Jr., Hernandez M, Lee JJ, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol (2019) 37(18):1558–65. 10.1200/JCO.19.00201 - DOI - PMC - PubMed
-
- Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet (2019) 394(10212):1915–28. 10.1016/S0140-6736(19)32591-7 - DOI - PubMed
-
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol (2019) 37(7):537–46. 10.1200/JCO.18.00149 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous