Novel Strategies for Efficient Production and Delivery of Live Biotherapeutics and Biotechnological Uses of Lactococcus lactis: The Lactic Acid Bacterium Model
- PMID: 33251190
- PMCID: PMC7672206
- DOI: 10.3389/fbioe.2020.517166
Novel Strategies for Efficient Production and Delivery of Live Biotherapeutics and Biotechnological Uses of Lactococcus lactis: The Lactic Acid Bacterium Model
Abstract
Lactic acid bacteria (LAB) are traditionally used in fermentation and food preservation processes and are recognized as safe for consumption. Recently, they have attracted attention due to their health-promoting properties; many species are already widely used as probiotics for treatment or prevention of various medical conditions, including inflammatory bowel diseases, infections, and autoimmune disorders. Some LAB, especially Lactococcus lactis, have been engineered as live vehicles for delivery of DNA vaccines and for production of therapeutic biomolecules. Here, we summarize work on engineering of LAB, with emphasis on the model LAB, L. lactis. We review the various expression systems for the production of heterologous proteins in Lactococcus spp. and its use as a live delivery system of DNA vaccines and for expression of biotherapeutics using the eukaryotic cell machinery. We have included examples of molecules produced by these expression platforms and their application in clinical disorders. We also present the CRISPR-Cas approach as a novel methodology for the development and optimization of food-grade expression of useful substances, and detail methods to improve DNA delivery by LAB to the gastrointestinal tract. Finally, we discuss perspectives for the development of medical applications of recombinant LABs involving animal model studies and human clinical trials, and we touch on the main safety issues that need to be taken into account so that bioengineered versions of these generally recognized as safe organisms will be considered acceptable for medical use.
Keywords: Lactococcus lactis; biotherapeutic molecules; genetic engineering; mucosal immunity; safe for consumption.
Copyright © 2020 Tavares, de Jesus, da Silva, Barroso, Batista, Coelho-Rocha, Azevedo, Drumond and Mancha-Agresti.
Figures
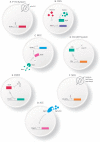
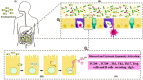
 ); (II) Bacteria in contact with the intestinal surface where they are recognized by the Microfold cells (M cells) (
); (II) Bacteria in contact with the intestinal surface where they are recognized by the Microfold cells (M cells) ( ), enterocytes (
), enterocytes ( ), or immune cells [such as dendritic cells (
), or immune cells [such as dendritic cells ( )]; (III) The recombinant bacteria is engulfed by the phagolysosome complex and lysed (
)]; (III) The recombinant bacteria is engulfed by the phagolysosome complex and lysed ( ). The vaccinal plasmid (
). The vaccinal plasmid ( ) escapes from the vesicle and reaches the nucleus (
) escapes from the vesicle and reaches the nucleus ( ) of the host cell. Inside the nucleus transcription of the gene of interest occurs (
) of the host cell. Inside the nucleus transcription of the gene of interest occurs ( ); (IV) The protein (
); (IV) The protein ( ) is exposed to the immune system, inducing cellular and humoral immune responses.
) is exposed to the immune system, inducing cellular and humoral immune responses.
Similar articles
-
Vector Development Timeline for Mucosal Vaccination and Treatment of Disease Using Lactococcus lactis and Design Approaches of Next Generation Food Grade Plasmids.Front Microbiol. 2018 Aug 14;9:1805. doi: 10.3389/fmicb.2018.01805. eCollection 2018. Front Microbiol. 2018. PMID: 30154762 Free PMC article. Review.
-
Heterologous protein production and delivery systems for Lactococcus lactis.Genet Mol Res. 2003 Mar 31;2(1):102-11. Genet Mol Res. 2003. PMID: 12917806 Review.
-
[Progress on lactococcus lactis expressing heterologous antigens as live mucosal vaccines].Wei Sheng Wu Xue Bao. 2006 Aug;46(4):680-3. Wei Sheng Wu Xue Bao. 2006. PMID: 17037080 Review. Chinese.
-
Engineering of lactic acid bacteria for delivery of therapeutic proteins and peptides.Appl Microbiol Biotechnol. 2019 Mar;103(5):2053-2066. doi: 10.1007/s00253-019-09628-y. Epub 2019 Jan 17. Appl Microbiol Biotechnol. 2019. PMID: 30656391 Review.
-
Lactococcus lactis as a live vector: heterologous protein production and DNA delivery systems.Protein Expr Purif. 2011 Oct;79(2):165-75. doi: 10.1016/j.pep.2011.06.005. Epub 2011 Jun 16. Protein Expr Purif. 2011. PMID: 21704169 Review.
Cited by
-
Significance of African Diets in Biotherapeutic Modulation of the Gut Microbiome.Bioinform Biol Insights. 2021 Apr 27;15:11779322211012697. doi: 10.1177/11779322211012697. eCollection 2021. Bioinform Biol Insights. 2021. PMID: 33994782 Free PMC article. Review.
-
Engineering receptor-binding domain and heptad repeat domains towards the development of multi-epitopes oral vaccines against SARS-CoV-2 variants.PLoS One. 2024 Aug 15;19(8):e0306111. doi: 10.1371/journal.pone.0306111. eCollection 2024. PLoS One. 2024. PMID: 39146295 Free PMC article.
-
The Presence of Plasmids in Lactococcus lactis IL594 Determines Changes in the Host Phenotype and Expression of Chromosomal Genes.Int J Mol Sci. 2023 Jan 2;24(1):793. doi: 10.3390/ijms24010793. Int J Mol Sci. 2023. PMID: 36614234 Free PMC article.
-
Growth differentiation factor 11 delivered by dairy Lactococcus lactis strains modulates inflammation and prevents mucosal damage in a mice model of intestinal mucositis.Front Microbiol. 2023 Apr 17;14:1157544. doi: 10.3389/fmicb.2023.1157544. eCollection 2023. Front Microbiol. 2023. PMID: 37138633 Free PMC article.
-
Identification, Characterization, and Epidemiological Analysis of Lactococcus garvieae Fish Isolates Obtained in a Period of Eighteen Years.Microorganisms. 2025 Feb 17;13(2):436. doi: 10.3390/microorganisms13020436. Microorganisms. 2025. PMID: 40005801 Free PMC article.
References
-
- Aliakbarpour H. R., Chamani M., Rahimi G., Sadeghi A. A., Qujeq D. (2012). The Bacillus subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian Austr. J. Anim. Sci. 25 1285–1293. 10.5713/ajas.2012.12110 - DOI - PMC - PubMed
-
- Bahey-El-Din M., Gahan C. G. M. (2010). Lactococcus lactis: from the dairy industry to antigen and therapeutic protein delivery. Discov. Med. 9 455–461. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

