Coxiella burnetii in Dromedary Camels (Camelus dromedarius): A Possible Threat for Humans and Livestock in North Africa and the Near and Middle East?
- PMID: 33251255
- PMCID: PMC7674558
- DOI: 10.3389/fvets.2020.558481
Coxiella burnetii in Dromedary Camels (Camelus dromedarius): A Possible Threat for Humans and Livestock in North Africa and the Near and Middle East?
Abstract
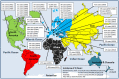
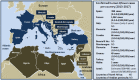
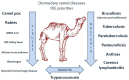
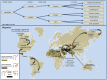
The "One Health" concept recognizes that human health is connected to animal health and to the ecosystems. Coxiella burnetii-induced human Q fever is one of the most widespread neglected zoonosis. The main animal reservoirs responsible for C. burnetii transmission to humans are domesticated ruminants, primarily goats, sheep, and cattle. Although studies are still too sparse to draw definitive conclusions, the most recent C. burnetii serosurvey studies conducted in herds and farms in Africa, North Africa, Arabian Peninsula, and Asia highlighted that seroprevalence was strikingly higher in dromedary camels (Camelus dromedarius) than in other ruminants. The C. burnetii seroprevalence in camel herds can reach more than 60% in Egypt, Saudi Arabia, and Sudan, and 70 to 80% in Algeria and Chad, respectively. The highest seroprevalence was in female camels with a previous history of abortion. Moreover, C. burnetii infection was reported in ticks of the Hyalomma dromedarii and Hyalomma impeltatum species collected on camels. Even if dromedary camels represent <3% of the domesticated ruminants in the countries of the Mediterranean basin Southern coast, these animals play a major socioeconomic role for millions of people who live in the arid zones of Africa, Middle East, and Asia. In Chad and Somalia, camels account for about 7 and 21% of domesticated ruminants, respectively. To meet the growing consumers demand of camel meat and milk (>5 million tons/year of both raw and pasteurized milk according to the Food and Agriculture Organization) sustained by a rapid increase of population (growth rate: 2.26-3.76 per year in North Africa), dromedary camel breeding tends to increase from the Maghreb to the Arabic countries. Because of possible long-term persistence of C. burnetii in camel hump adipocytes, this pathogen could represent a threat for herds and breeding farms and ultimately for public health. Because this review highlights a hyperendemia of C. burnetii in dromedary camels, a proper screening of herds and breeding farms for C. burnetii is urgently needed in countries where camel breeding is on the rise. Moreover, the risk of C. burnetii transmission from camel to human should be further evaluated.
Keywords: Coxiella burnetii; dromedary camel (Camelus dromedarius); epidemiology; human—animal coexistence; zoonoses awareness.
Copyright © 2020 Devaux, Osman, Million and Raoult.
Figures



References
-
- Babudieri B. Q fever: a zoonosis. Adv Vet Sci. (1959) 5:81–182.
Publication types
LinkOut - more resources
Full Text Sources

