Aspiration-assisted freeform bioprinting of prefabricated tissue spheroids in a yield-stress gel
- PMID: 33251340
- PMCID: PMC7695349
- DOI: 10.1038/s42005-020-00449-4
Aspiration-assisted freeform bioprinting of prefabricated tissue spheroids in a yield-stress gel
Abstract
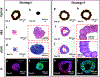
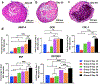
Bioprinting of cellular aggregates, such as tissue spheroids, to form three-dimensional (3D) complex-shaped arrangements, has posed a major challenge due to lack of robust, reproducible and practical bioprinting techniques. Here, we demonstrate 3D aspiration-assisted freeform bioprinting of tissue spheroids by precisely positioning them in self-healing yield-stress gels, enabling the self-assembly of spheroids for fabrication of tissues. The presented approach enables the traverse of spheroids directly from the cell media to the gel and freeform positioning of the spheroids on demand. We study the underlying physical mechanism of the approach to elucidate the interactions between the aspirated spheroids and the gel's yield-stress during the transfer of spheroids from cell media to the gel. We further demonstrate the application of the proposed approach in the realization of various freeform shapes and self-assembly of human mesenchymal stem cell spheroids for the construction of cartilage and bone tissues.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures





Similar articles
-
Aspiration-assisted freeform bioprinting of mesenchymal stem cell spheroids within alginate microgels.Biofabrication. 2022 Feb 8;14(2):10.1088/1758-5090/ac4dd8. doi: 10.1088/1758-5090/ac4dd8. Biofabrication. 2022. PMID: 35062000 Free PMC article.
-
Aspiration-assisted bioprinting for precise positioning of biologics.Sci Adv. 2020 Mar 6;6(10):eaaw5111. doi: 10.1126/sciadv.aaw5111. eCollection 2020 Mar. Sci Adv. 2020. PMID: 32181332 Free PMC article.
-
Aspiration-assisted bioprinting of co-cultured osteogenic spheroids for bone tissue engineering.Biofabrication. 2020 Dec 17;13(1). doi: 10.1088/1758-5090/abc1bf. Biofabrication. 2020. PMID: 33059343
-
Strategies for 3D bioprinting of spheroids: A comprehensive review.Biomaterials. 2022 Dec;291:121881. doi: 10.1016/j.biomaterials.2022.121881. Epub 2022 Oct 28. Biomaterials. 2022. PMID: 36335718 Review.
-
Cell spheroids as a versatile research platform: formation mechanisms, high throughput production, characterization and applications.Biofabrication. 2021 Apr 8;13(3). doi: 10.1088/1758-5090/abe6f2. Biofabrication. 2021. PMID: 33592595 Review.
Cited by
-
Complex 3D bioprinting methods.APL Bioeng. 2021 Mar 11;5(1):011508. doi: 10.1063/5.0034901. eCollection 2021 Mar. APL Bioeng. 2021. PMID: 33728391 Free PMC article. Review.
-
Review on Bioinspired Design of ECM-Mimicking Scaffolds by Computer-Aided Assembly of Cell-Free and Cell Laden Micro-Modules.J Funct Biomater. 2023 Feb 13;14(2):101. doi: 10.3390/jfb14020101. J Funct Biomater. 2023. PMID: 36826900 Free PMC article. Review.
-
miRNA induced 3D bioprinted-heterotypic osteochondral interface.Biofabrication. 2022 Aug 17;14(4):10.1088/1758-5090/ac7fbb. doi: 10.1088/1758-5090/ac7fbb. Biofabrication. 2022. PMID: 35803212 Free PMC article.
-
Embedded 3D Printing in Self-Healing Annealable Composites for Precise Patterning of Functionally Mature Human Neural Constructs.Adv Sci (Weinh). 2022 Sep;9(25):e2201392. doi: 10.1002/advs.202201392. Epub 2022 Jun 16. Adv Sci (Weinh). 2022. PMID: 35712780 Free PMC article.
-
3D cell aggregate printing technology and its applications.Essays Biochem. 2021 Aug 10;65(3):467-480. doi: 10.1042/EBC20200128. Essays Biochem. 2021. PMID: 34223609 Free PMC article. Review.
References
-
- Dababneh AB & Ozbolat IT Bioprinting technology: a current state-ofthe-art review. J. Manuf. Sci. Eng 136, 061016 (2014).
-
- Sun W et al. The bioprinting roadmap. Biofabrication 12, 022002 (2020). - PubMed
-
- Ozbolat IT 3D Bioprinting: Fundamentals, Principles and Applications (Academic Press, London, 2016).
Grants and funding
LinkOut - more resources
Full Text Sources
