Efficient Chemical Synthesis of (Epi)catechin Glucuronides: Brain-Targeted Metabolites for Treatment of Alzheimer's Disease and Other Neurological Disorders
- PMID: 33251444
- PMCID: PMC7689943
- DOI: 10.1021/acsomega.0c04512
Efficient Chemical Synthesis of (Epi)catechin Glucuronides: Brain-Targeted Metabolites for Treatment of Alzheimer's Disease and Other Neurological Disorders
Abstract
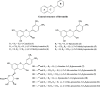
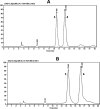
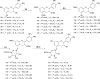
Grape seed extract (GSE) is rich in flavonoids and has been recognized to possess human health benefits. Our group and others have demonstrated that GSE is able to attenuate the development of Alzheimer's disease (AD). Moreover, our results have disclosed that the anti-Alzheimer's benefits are not directly/solely related to the dietary flavonoids themselves, but rather to their metabolites, particularly to the glucuronidated ones. To facilitate the understanding of regioisomer/stereoisomer-specific biological effects of (epi)catechin glucuronides, we here describe a concise chemical synthesis of authentic standards of catechin and epicatechin metabolites 3-12. The synthesis of glucuronides 9 and 12 is described here for the first time. The key reactions employed in the synthesis of the novel glucuronides 9 and 12 include the regioselective methylation of the 4'-hydroxyl group of (epi)catechin (≤1.0/99.0%; 3'-OMe/4'-OMe) and the regioselective deprotection of the tert-butyldimethylsilyl (TBS) group at position 5 (yielding up to 79%) over the others (3, 7 and 3' or 4').
© 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


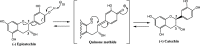
Similar articles
-
Ovariectomy lowers urine levels of unconjugated (+)-catechin, (-)-epicatechin, and their methylated metabolites in rats fed grape seed extract.Horm Mol Biol Clin Investig. 2013 Dec;16(3):129-38. doi: 10.1515/hmbci-2013-0044. Horm Mol Biol Clin Investig. 2013. PMID: 25436866
-
Synthesis and quantitative analysis of plasma-targeted metabolites of catechin and epicatechin.J Agric Food Chem. 2015 Mar 4;63(8):2233-40. doi: 10.1021/jf505922b. Epub 2015 Feb 20. J Agric Food Chem. 2015. PMID: 25671729
-
Enzymatic synthesis of substituted epicatechins for bioactivity studies in neurological disorders.Biochem Biophys Res Commun. 2012 Jan 6;417(1):457-61. doi: 10.1016/j.bbrc.2011.11.139. Epub 2011 Dec 7. Biochem Biophys Res Commun. 2012. PMID: 22166210 Free PMC article.
-
Glucuronidated Flavonoids in Neurological Protection: Structural Analysis and Approaches for Chemical and Biological Synthesis.J Agric Food Chem. 2017 Sep 6;65(35):7607-7623. doi: 10.1021/acs.jafc.7b02633. Epub 2017 Aug 21. J Agric Food Chem. 2017. PMID: 28789524 Free PMC article. Review.
-
Procyanidins and Alzheimer's Disease.Mol Neurobiol. 2019 Aug;56(8):5556-5567. doi: 10.1007/s12035-019-1469-6. Epub 2019 Jan 16. Mol Neurobiol. 2019. PMID: 30649713 Review.
Cited by
-
Glucuronic acid metabolites of phenolic acids target AKT-PH domain to improve glucose metabolism.Chin Herb Med. 2023 Apr 12;15(3):398-406. doi: 10.1016/j.chmed.2022.11.005. eCollection 2023 Jul. Chin Herb Med. 2023. PMID: 37538860 Free PMC article.
-
Antifungal compounds of Chinese prickly ash against drug-resistant Candida albicans.Food Chem X. 2022 Jul 25;15:100400. doi: 10.1016/j.fochx.2022.100400. eCollection 2022 Oct 30. Food Chem X. 2022. PMID: 36211763 Free PMC article.
-
UGT84F9 is the major flavonoid UDP-glucuronosyltransferase in Medicago truncatula.Plant Physiol. 2021 Apr 23;185(4):1617-1637. doi: 10.1093/plphys/kiab016. Plant Physiol. 2021. PMID: 33694362 Free PMC article.
-
(±)-Catechin-A Mass-Spectrometry-Based Exploration Coordination Complex Formation with FeII and FeIII.Cells. 2022 Mar 11;11(6):958. doi: 10.3390/cells11060958. Cells. 2022. PMID: 35326409 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

