Towards better understanding of the influence of process parameters in roll compaction/dry granulation on throughput, ribbon microhardness and granule failure load
- PMID: 33251510
- PMCID: PMC7679258
- DOI: 10.1016/j.ijpx.2020.100059
Towards better understanding of the influence of process parameters in roll compaction/dry granulation on throughput, ribbon microhardness and granule failure load
Abstract
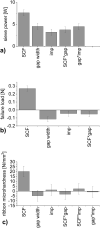
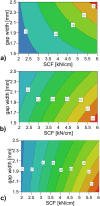
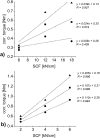
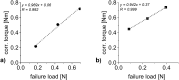
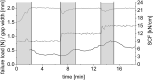
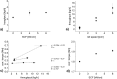
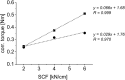
A key quality attribute for solid oral dosage forms is their hardness and ability to withstand breaking or grinding. If the product is to be manufactured continuously, it can be of interest to monitor the hardness of the material at different stages of manufacturing. Using the controlled process parameters of roll compaction/dry granulation specific compaction force, roll speed and gap width, hardness of the resulting ribbons and granules can be predicted. For the first time, in this study two yield variables (corrected torque of the granulation unit and throughput of material) are used to predict the granules failure load. The increase in granule hardness was monitored in-line when the specific compaction force was increased during the compaction process. This opens the way for in-line control of material hardness, and its use for feedback and feedforward control loops for future continuous manufacturing processes.
Keywords: Continuous manufacturing; Granule strength/failure load; Microhardness; Process analytical technologies; Process monitoring; Roll compaction/dry granulation.
© 2020 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Adams M.J., Mullier M.A., Seville J.P.K. Agglomerate strength measurement using a uniaxial confined compression test. Powder Technol. 1994;78:5–13.
-
- Arndt O.-R., Baggio R., Adam A.K., Harting J., Franceschinis E., Kleinebudde P. Impact of different dry and wet granulation techniques on granule and tablet properties: a comparative study. J. Pharm. Sci. 2018;107:3143–3152. - PubMed
-
- Broitman E. Indentation hardness measurements at macro-, micro-, and nanoscale: a critical overview. Ribol. Lett. 2017;65 (article number 23)
-
- Chatterjee S. IFPAC Annual Meeting; Baltimore: 2012. FDA Perspective on Continuous Manufacturing.
-
- Chowhan Z. Moisture, hardness, disintegration and dissolution interrelationships in compressed tablets prepared by the wet granulation process. Drug Dev. Ind. Pharm. 1979;5:41–62.
LinkOut - more resources
Full Text Sources
Miscellaneous

