A Computational and Experimental Investigation of the Origin of Selectivity in the Chiral Phosphoric Acid Catalyzed Enantioselective Minisci Reaction
- PMID: 33252228
- PMCID: PMC7747223
- DOI: 10.1021/jacs.0c09668
A Computational and Experimental Investigation of the Origin of Selectivity in the Chiral Phosphoric Acid Catalyzed Enantioselective Minisci Reaction
Abstract
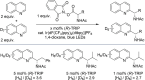
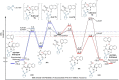
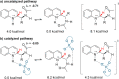
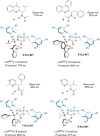
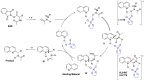
The Minisci reaction is one of the most valuable methods for directly functionalizing basic heteroarenes to form carbon-carbon bonds. Use of prochiral, heteroatom-substituted radicals results in stereocenters being formed adjacent to the heteroaromatic system, generating motifs which are valuable in medicinal chemistry and chiral ligand design. Recently a highly enantioselective and regioselective protocol for the Minisci reaction was developed, using chiral phosphoric acid catalysis. However, the precise mechanism by which this process operated and the origin of selectivity remained unclear, making it challenging to develop the reaction more generally. Herein we report further experimental mechanistic studies which feed into detailed DFT calculations that probe the precise nature of the stereochemistry-determining step. Computational and experimental evidence together support Curtin-Hammett control in this reaction, with initial radical addition being quick and reversible, and enantioselectivity being achieved in the subsequent slower, irreversible deprotonation. A detailed survey via DFT calculations assessed a number of different possibilities for selectivity-determining deprotonation of the radical cation intermediate. Computations point to a clear preference for an initially unexpected mode of internal deprotonation enacted by the amide group, which is a crucial structural feature of the radical precursor, with the assistance of the associated chiral phosphate. This unconventional stereodetermining step underpins the high enantioselectivities and regioselectivities observed. The mechanistic model was further validated by applying it to a test set of substrates possessing varied structural features.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Minisci F.; Vismara E.; Fontana F. Recent Developments of Free-Radical Substitutions of Heteroaromatic Bases. Heterocycles 1989, 28, 489–519. 10.3987/REV-88-SR1. - DOI
- Minisci F.; Fontana F.; Vismara E. Substitutions by nucleophilic free radicals: A new general reaction of heteroaromatic bases. J. Heterocycl. Chem. 1990, 27, 79–96. 10.1002/jhet.5570270107. - DOI
- Duncton M. A. J. Minisci reactions: Versatile CH-functionalizations for medicinal chemists. MedChemComm 2011, 2, 1135–1161. 10.1039/c1md00134e. - DOI
- Tauber J.; Imbri D.; Opatz T. Radical Addition to Iminium Ions and Cationic Heterocycles. Molecules 2014, 19, 16190.10.3390/molecules191016190. - DOI - PMC - PubMed
- Proctor R. S. J.; Phipps R. J. Recent Advances in Minisci-Type Reactions. Angew. Chem., Int. Ed. 2019, 58, 13666–13699. 10.1002/anie.201900977. - DOI - PubMed
-
- Uraguchi D.; Terada M. Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation. J. Am. Chem. Soc. 2004, 126, 5356–5357. 10.1021/ja0491533. - DOI - PubMed
- Akiyama T.; Itoh J.; Yokota K.; Fuchibe K. Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid. Angew. Chem., Int. Ed. 2004, 43, 1566–1568. 10.1002/anie.200353240. - DOI - PubMed
- Parmar D.; Sugiono E.; Raja S.; Rueping M. Complete Field Guide to Asymmetric BINOL-Phosphate Derived Brønsted Acid and Metal Catalysis: History and Classification by Mode of Activation; Brønsted Acidity, Hydrogen Bonding, Ion Pairing, and Metal Phosphates. Chem. Rev. 2014, 114, 9047–9153. 10.1021/cr5001496. - DOI - PubMed
-
-
For examples of CPAs in radical chemistry, see:
- Rono L. J.; Yayla H. G.; Wang D. Y.; Armstrong M. F.; Knowles R. R. Enantioselective Photoredox Catalysis Enabled by Proton-Coupled Electron Transfer: Development of an Asymmetric Aza-Pinacol Cyclization. J. Am. Chem. Soc. 2013, 135, 17735–17738. 10.1021/ja4100595. - DOI - PubMed
- Lin J.-S.; Dong X.-Y.; Li T.-T.; Jiang N.-C.; Tan B.; Liu X.-Y. A Dual-Catalytic Strategy To Direct Asymmetric Radical Aminotrifluoromethylation of Alkenes. J. Am. Chem. Soc. 2016, 138, 9357–9360. 10.1021/jacs.6b04077. - DOI - PubMed
- Hepburn H. B.; Melchiorre P. Brønsted acid-catalysed conjugate addition of photochemically generated α-amino radicals to alkenylpyridines. Chem. Commun. 2016, 52, 3520–3523. 10.1039/C5CC10401G. - DOI - PubMed
- Wang F.-L.; Dong X.-Y.; Lin J.-S.; Zeng Y.; Jiao G.-Y.; Gu Q.-S.; Guo X.-Q.; Ma C.-L.; Liu X.-Y. Catalytic Asymmetric Radical Diamination of Alkenes. Chem. 2017, 3, 979–990. 10.1016/j.chempr.2017.10.008. - DOI
- Lin L.; Bai X.; Ye X.; Zhao X.; Tan C.-H.; Jiang Z. Organocatalytic Enantioselective Protonation for Photoreduction of Activated Ketones and Ketimines Induced by Visible Light. Angew. Chem., Int. Ed. 2017, 56, 13842–13846. 10.1002/anie.201707899. - DOI - PubMed
- Li J.; Kong M.; Qiao B.; Lee R.; Zhao X.; Jiang Z. Formal enantioconvergent substitution of alkyl halides via catalytic asymmetric photoredox radical coupling. Nat. Commun. 2018, 9, 2445.10.1038/s41467-018-04885-3. - DOI - PMC - PubMed
- Gentry E. C.; Rono L. J.; Hale M. E.; Matsuura R.; Knowles R. R. Enantioselective Synthesis of Pyrroloindolines via Noncovalent Stabilization of Indole Radical Cations and Applications to the Synthesis of Alkaloid Natural Products. J. Am. Chem. Soc. 2018, 140, 3394–3402. 10.1021/jacs.7b13616. - DOI - PMC - PubMed
- Yin Y.; Dai Y.; Jia H.; Li J.; Bu L.; Qiao B.; Zhao X.; Jiang Z. Conjugate Addition–Enantioselective Protonation of N-Aryl Glycines to α-Branched 2-Vinylazaarenes via Cooperative Photoredox and Asymmetric Catalysis. J. Am. Chem. Soc. 2018, 140, 6083–6087. 10.1021/jacs.8b01575. - DOI - PubMed
- Morse P. D.; Nguyen T. M.; Cruz C. L.; Nicewicz D. A. Enantioselective counter-anions in photoredox catalysis: The asymmetric cation radical Diels-Alder reaction. Tetrahedron 2018, 74, 3266–3272. 10.1016/j.tet.2018.03.052. - DOI - PMC - PubMed
- Cao K.; Tan S. M.; Lee R.; Yang S.; Jia H.; Zhao X.; Qiao B.; Jiang Z. Catalytic Enantioselective Addition of Prochiral Radicals to Vinylpyridines. J. Am. Chem. Soc. 2019, 141, 5437–5443. 10.1021/jacs.9b00286. - DOI - PubMed
- Shin N. Y.; Ryss J. M.; Zhang X.; Miller S. J.; Knowles R. R. Light driven deracemization enabled by excited state electron transfer. Science 2019, 366, 364–369. 10.1126/science.aay2204. - DOI - PMC - PubMed
- Lin J.-S.; Li T.-T.; Liu J.-R.; Jiao G.-Y.; Gu Q.-S.; Cheng J.-T.; Guo Y.-L.; Hong X.; Liu X.-Y. Cu/Chiral Phosphoric Acid-Catalyzed Asymmetric Three-Component Radical-Initiated 1,2-Dicarbofunctionalization of Alkenes. J. Am. Chem. Soc. 2019, 141, 1074–1083. 10.1021/jacs.8b11736. - DOI - PubMed
- Roos C. B.; Demaerel J.; Graff D. E.; Knowles R. R. Enantioselective Hydroamination of Alkenes with Sulfonamides Enabled by Proton-Coupled Electron Transfer. J. Am. Chem. Soc. 2020, 142, 5974–5979. 10.1021/jacs.0c01332. - DOI - PMC - PubMed
- Ye L.; Tian Y.; Meng X.; Gu Q.-S.; Liu X.-Y. Enantioselective Copper(I)/Chiral Phosphoric Acid Catalyzed Intramolecular Amination of Allylic and Benzylic C-H Bonds. Angew. Chem., Int. Ed. 2020, 59, 1129–1133. 10.1002/anie.201911742. - DOI - PubMed
-
Publication types
LinkOut - more resources
Full Text Sources

