CT-guided 125I brachytherapy combined with chemotherapy for the treatment of unresectable or locally advanced pancreatic carcinoma
- PMID: 33252336
- PMCID: PMC7837730
- DOI: 10.5152/dir.2020.19371
CT-guided 125I brachytherapy combined with chemotherapy for the treatment of unresectable or locally advanced pancreatic carcinoma
Abstract
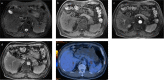
Purpose: We aimed to explore the feasibility and clinical effectiveness of percutaneous CT-guided iodine-125 (¹²⁵I) brachytherapy combined with chemotherapy for the treatment of patients with unresectable or locally advanced pancreatic carcinoma (PC).
Methods: We retrospectively reviewed 66 patients with Stage III and IV PC who had received chemotherapy. A total of 35 (53%) patients receiving 125I brachytherapy and chemotherapy (gemcitabine + cisplatin, GP) were classified as Group A, and 31 (47%) patients who received GP chemotherapy alone were categorized as Group B. The evaluated indications were local control rate (LCR), local progression-free survival (LPFS), overall survival (OS), treatment-related complications, and the degree of symptom relief. Kaplan-Meier curves, log-rank test and Cox regression models were generated and used for further analysis to identify predictors of outcomes.
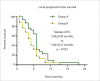
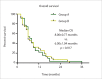
Results: The median follow-up time was 6.00±0.84 months. The 1-, 3-, 6-, 12- and 18-month LCRs for Group A were 100% (35/35), 89.3% (25/28), 71.4% (15/21), 37.5% (3/8) and 33.3% (1/3), respectively; and those for Group B were 87.1% (27/31), 69.6% (16/23), 41.2% (7/17), 14.3% (1/7) and 0% (0/3), respectively. The LCR differed at 1-, 3- and 6-months (P = 0.032; P = 0.009; P = 0.030; respectively). The median LPFS was 7.00±0.30 months and 5.00±0.75 months for Groups A and B (P = 0.023), respectively; however, the median OS of the groups were not significantly different (8.00±0.77 months vs. 6.00±1.04 months. P = 0.917). No life-threatening complications occurred during or after the procedures. Patients in Group A experienced better pain control and relief of abdominal distension than those in Group B.
Conclusion: CT-guided 125I brachytherapy is a feasible, safe, and valuable treatment for patients with unresectable PC.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures



Similar articles
-
Intratumoral injection therapies for locally advanced pancreatic cancer: systematic review.BJS Open. 2023 May 5;7(3):zrad052. doi: 10.1093/bjsopen/zrad052. BJS Open. 2023. PMID: 37254902 Free PMC article.
-
Feasibility of 125I brachytherapy combined with arterial infusion chemotherapy in patients with advanced pancreatic cancer.Medicine (Baltimore). 2023 Nov 3;102(44):e35033. doi: 10.1097/MD.0000000000035033. Medicine (Baltimore). 2023. PMID: 37933058 Free PMC article.
-
Feasibility and clinical value of CT-guided 125I brachytherapy for metastatic soft tissue sarcoma after first-line chemotherapy failure.Eur Radiol. 2018 Mar;28(3):1194-1203. doi: 10.1007/s00330-017-5036-0. Epub 2017 Sep 27. Eur Radiol. 2018. PMID: 28956119
-
Percutaneous computed tomography-guided iodine-125 seeds implantation for unresectable pancreatic cancer.Indian J Cancer. 2015 Dec;52 Suppl 2:e69-74. doi: 10.4103/0019-509X.172517. Indian J Cancer. 2015. PMID: 26728678
-
Interstitial brachytherapy for pancreatic cancer: report of seven cases treated with 125I and a review of the literature.Int J Radiat Oncol Biol Phys. 1991 Jul;21(2):451-7. doi: 10.1016/0360-3016(91)90795-6. Int J Radiat Oncol Biol Phys. 1991. PMID: 2061121 Review.
Cited by
-
Intratumoral injection therapies for locally advanced pancreatic cancer: systematic review.BJS Open. 2023 May 5;7(3):zrad052. doi: 10.1093/bjsopen/zrad052. BJS Open. 2023. PMID: 37254902 Free PMC article.
-
Prognostic Evaluation of CT Imaging Big Data-Assisted Arterial Chemoembolization Combined with 125I Seed Implantation for Non-Small-Cell Lung Cancer.Comput Math Methods Med. 2022 Jul 13;2022:3472982. doi: 10.1155/2022/3472982. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35872936 Free PMC article.
-
Spinal brachytherapy.Neuro Oncol. 2022 Nov 2;24(Suppl 6):S62-S68. doi: 10.1093/neuonc/noac094. Neuro Oncol. 2022. PMID: 36322097 Free PMC article.
-
Iodine-125 seed implantation in the treatment of malignant tumors.J Interv Med. 2023 Jul 22;6(3):111-115. doi: 10.1016/j.jimed.2023.07.006. eCollection 2023 Aug. J Interv Med. 2023. PMID: 37846333 Free PMC article.
-
125I Intracavitary Irradiation Combined with 125I Seeds Implantation for Treatment of Locally Advanced Pancreatic Head Cancer: A Retrospective Analysis of 67 Cases.Int J Gen Med. 2021 Jun 18;14:2645-2653. doi: 10.2147/IJGM.S309069. eCollection 2021. Int J Gen Med. 2021. PMID: 34177273 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

