Pharmacokinetics and safety of maraviroc in neonates
- PMID: 33252481
- PMCID: PMC7856036
- DOI: 10.1097/QAD.0000000000002762
Pharmacokinetics and safety of maraviroc in neonates
Abstract
Objective: The aim of this study was to evaluate safety and pharmacokinetics of maraviroc administered with standard antiretroviral prophylaxis to HIV-1 exposed infants and to determine the appropriate dose of maraviroc during the first 6 weeks of life.
Design: A phase I, multicentre, open-label study enrolling two sequential cohorts.
Methods: IMPAACT 2007 participants enrolled by day 3 of life and were stratified by exposure to maternal efavirenz. Cohort 1 participants received two single 8 mg/kg maraviroc doses 1 week apart with pharmacokinetic sampling after each dose. Cohort 2 participants received 8 mg/kg maraviroc twice daily through 6 weeks of life with pharmacokinetic sampling at weeks 1 and 4. Maraviroc exposure target was Cavg at least 75 ng/ml. Laboratory and clinical evaluations assessed safety.
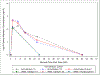
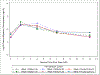
Results: Fifteen Cohort 1 and 32 Cohort 2 HIV-exposed neonates were enrolled (median gestational age 39 weeks, 51% male). All 13 evaluable Cohort 1 infants met the pharmacokinetic target. Median exposure for the 25 evaluable Cohort 2 infants met the pharmacokinetic target but variability was high, with 17-33% of infants below target at Weeks 1 and 4. Pharmacokinetic target achievement was similar between efavirenz exposure strata. No Grade 3+ toxicities, early study or treatment discontinuations due to maraviroc occurred.
Conclusion: Median maraviroc exposure met the Cavg target in neonates receiving 8 mg/kg twice daily, although exposures were variable. Maternal efavirenz use did not impact maraviroc exposure and no discontinuations were due to maraviroc toxicity/intolerance. No infants acquired HIV-1 infection during follow-up. Maraviroc 8 mg/kg twice daily appears well tolerated during the first 6 weeks of life.
Copyright © 2020 Wolters Kluwer Health, Inc. All rights reserved.
Figures


References
-
- UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet. Available from: https://www.unaids.org/en/resources/fact-sheet.
-
- Smits A, Annaert P, Allegaert K. Drug disposition and clinical practice in neonates: cross talk between developmental physiology and pharmacology. Int J Pharm 2013; 452(1–2):8–13. - PubMed
-
- Clarke DF, Penazzato M, Capparelli E, Cressey TR, Siberry G, Sugandhi N, et al. Prevention and treatment of HIV infection in neonates: evidence base for existing WHO dosing recommendations and implementation considerations. Expert Rev Clin Pharmacol 2018; 11(1):83–93. - PubMed

