Protocols for Experimental Sjögren's Syndrome
- PMID: 33252847
- PMCID: PMC8959043
- DOI: 10.1002/cpim.114
Protocols for Experimental Sjögren's Syndrome
Abstract
Sjögren's syndrome (SS) is a systemic autoimmune disease affecting multiple organ systems. Salivary and lacrimal gland involvement cause dry mouth and dry eye and are the most common clinical presentations of the disease. Patients with SS also have autoantibodies targeting multiple nuclear and cytoplasmic antigens. Innate immune activation plays a critical role in SS pathogenesis. This article describes the activation of specific innate immune pathways in mice to study SS salivary gland manifestations. Methodologies for evaluating salivary gland inflammation and salivary function are described. This article also describes protocols for in-house assays to measure autoantibody titers in serum. © 2020 Wiley Periodicals LLC Basic Protocol 1: Acceleration of Sjögren's syndrome by activating the toll-like receptor 3 pathway Basic Protocol 2: Induction of Sjögren's syndrome by activating the stimulator of interferon genes pathway Alternate Protocol: Acceleration of Sjögren's syndrome by the administration of Freund's incomplete adjuvant Support Protocol 1: Evaluating salivary gland function Support Protocol 2: Evaluating salivary gland inflammation Support Protocol 3: Measuring autoantibody titers by indirect immunofluorescence.
Keywords: STING; Sjögren's syndrome; TLR3; innate immunity; salivary glands.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Figures


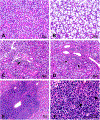
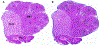

References
-
- Amador-Patarroyo MJ, Arbelaez JG, Mantilla RD, Rodriguez-Rodriguez A, Cárdenas-Roldán J, Pineda-Tamayo R, Guarin MR, Kleine LL, Rojas-Villarraga A, & Anaya JM (2012). Sjögren’s syndrome at the crossroad of polyautoimmunity. Journal of autoimmunity, 39(3), 199–205. - PubMed
-
- Cha S, Nagashima H, Brown VB, Peck AB, & Humphreys-Beher MG (2002). Two NOD Idd-associated intervals contribute synergistically to the development of autoimmune exocrinopathy (Sjögren’s syndrome) on a healthy murine background. Arthritis and rheumatism, 46(5), 1390–1398. - PubMed
-
- Chan EK, Damoiseaux J, Carballo OG, Conrad K, de Melo Cruvinel W, Francescantonio PL, Fritzler MJ, Garcia-De La Torre I, Herold M, Mimori T, Satoh M, von Mühlen CA, & Andrade LE (2015). Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014–2015. Frontiers in immunology, 6, 412. 10.3389/fimmu.2015.00412 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

