Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide
- PMID: 33252860
- PMCID: PMC7668192
- DOI: 10.1002/ctm2.228
Cytosolic DNA-STING-NLRP3 axis is involved in murine acute lung injury induced by lipopolysaccharide
Abstract
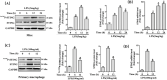
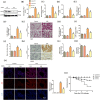
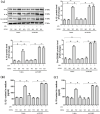
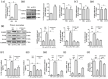
The role of NOD-like receptor protein 3 (NLRP3)-mediated pyroptosis in acute lung injury (ALI) has been well identified previously. Stimulator of interferon genes (STING) is an indispensable adaptor protein, which could regulate inflammation and pyroptosis during infection; however, its role in lipopolysaccharide (LPS)-induced ALI remains obscure. This study aimed to explore whether STING participated in the development of LPS-induced ALI as well as the underlying mechanism. We confirmed that LPS significantly enhanced the expression and phosphorylation of STING in lung tissue and primary macrophages from mice. STING deficiency relieved inflammation and oxidative stress in LPS-treated murine lungs and macrophages. Meanwhile, STING deficiency also abolished the activation of NLRP3 inflammasome and pyroptosis; however, NLRP3 overexpression by adenovirus offset the beneficial effects of STING deficiency in macrophages treated with LPS. Additionally, the level of mitochondrial DNA (mt-DNA) significantly increased in macrophages after LPS treatment. Intriguingly, although exogenous mt-DNA stimulation did not influence the level of STING, it could still trigger the phosphorylation of STING as well as pyroptosis, inflammation, and oxidative stress of macrophages. And the adverse effects induced by mt-DNA could be offset after STING was knocked out. Furthermore, the inhibition of the sensory receptor of cytosolic DNA (cyclic GMP-AMP synthase, cGAS) also blocked the activation of STING and NLRP3 inflammasome, meanwhile, it alleviated ALI without affecting the expression of STING after LPS challenge. Furthermore, cGAS inhibition also blocked the production of cGAMP induced by LPS, indicating that mt-DNA and cGAS could activate STING-NLRP3-mediated pyroptosis independent of the expression of STING. Finally, we found that LPS upregulated the expression of transcription factor c-Myc, which subsequently enhanced the activity of STING promoter and promoted its expression without affecting its phosphorylation. Collectively, our study disclosed that LPS could activate STING in a cytosolic DNA-dependent manner and upregulate the expression of STING in a c-Myc-dependent manner, which cooperatively contribute to ALI.
Keywords: NLRP3; STING; acute lung injury; cytosolic DNA; sepsis.
© 2020 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Fleischmann C, Scherag A, Adhikari NKJ, et al. Assessment of global incidence and mortality of hospital‐treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259‐272. - PubMed
-
- Eubank TA, Long SW, Perez KK. Role of rapid diagnostics in diagnosis and management of patients with sepsis. J Infect Dis. 2020;222:S103‐S109. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
