Genomic rearrangements uncovered by genome-wide co-evolution analysis of a major nosocomial pathogen, Enterococcus faecium
- PMID: 33253085
- PMCID: PMC8116687
- DOI: 10.1099/mgen.0.000488
Genomic rearrangements uncovered by genome-wide co-evolution analysis of a major nosocomial pathogen, Enterococcus faecium
Abstract
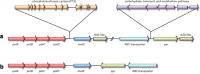
Enterococcus faecium is a gut commensal of the gastro-digestive tract, but also known as nosocomial pathogen among hospitalized patients. Population genetics based on whole-genome sequencing has revealed that E. faecium strains from hospitalized patients form a distinct clade, designated clade A1, and that plasmids are major contributors to the emergence of nosocomial E. faecium. Here we further explored the adaptive evolution of E. faecium using a genome-wide co-evolution study (GWES) to identify co-evolving single-nucleotide polymorphisms (SNPs). We identified three genomic regions harbouring large numbers of SNPs in tight linkage that are not proximal to each other based on the completely assembled chromosome of the clade A1 reference hospital isolate AUS0004. Close examination of these regions revealed that they are located at the borders of four different types of large-scale genomic rearrangements, insertion sites of two different genomic islands and an IS30-like transposon. In non-clade A1 isolates, these regions are adjacent to each other and they lack the insertions of the genomic islands and IS30-like transposon. Additionally, among the clade A1 isolates there is one group of pet isolates lacking the genomic rearrangement and insertion of the genomic islands, suggesting a distinct evolutionary trajectory. In silico analysis of the biological functions of the genes encoded in three regions revealed a common link to a stress response. This suggests that these rearrangements may reflect adaptation to the stringent conditions in the hospital environment, such as antibiotics and detergents, to which bacteria are exposed. In conclusion, to our knowledge, this is the first study using GWES to identify genomic rearrangements, suggesting that there is considerable untapped potential to unravel hidden evolutionary signals from population genomic data.
Keywords: Enterococcus faecium; genome-wide co-evolution analysis; genomic rearrangement.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
-
- Weiner LM, Webb AK, Limbago B, Dudeck MA, Patel J, et al. Antimicrobial-Resistant pathogens associated with healthcare-associated infections: summary of data reported to the National healthcare safety network at the centers for disease control and prevention, 2011-2014. Infect Control Hosp Epidemiol. 2016;37:1288–1301. doi: 10.1017/ice.2016.174. - DOI - PMC - PubMed
-
- Lebreton F, Willems RJL, Gilmore MS. Enterococcus diversity, origins in nature, and gut colonization. In: Enterococci: from commensals to leading causes of drug resistant infection [Internet] 2014. pp. 1–56.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

