Pre-existing resistance in the latent reservoir can compromise VRC01 therapy during chronic HIV-1 infection
- PMID: 33253162
- PMCID: PMC7728175
- DOI: 10.1371/journal.pcbi.1008434
Pre-existing resistance in the latent reservoir can compromise VRC01 therapy during chronic HIV-1 infection
Abstract
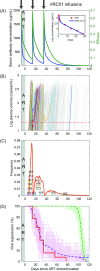
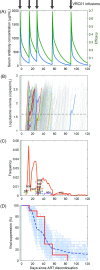
Passive immunization with broadly neutralizing antibodies (bNAbs) of HIV-1 appears a promising strategy for eliciting long-term HIV-1 remission. When administered concomitantly with the cessation of antiretroviral therapy (ART) to patients with established viremic control, bNAb therapy is expected to prolong remission. Surprisingly, in clinical trials on chronic HIV-1 patients, the bNAb VRC01 failed to prolong remission substantially. Identifying the cause of this failure is important for improving VRC01-based therapies and unraveling potential vulnerabilities of other bNAbs. In the trials, viremia resurged rapidly in most patients despite suppressive VRC01 concentrations in circulation, suggesting that VRC01 resistance was the likely cause of failure. ART swiftly halts viral replication, precluding the development of resistance during ART. If resistance were to emerge post ART, virological breakthrough would have taken longer than without VRC01 therapy. We hypothesized therefore that VRC01-resistant strains must have been formed before ART initiation, survived ART in latently infected cells, and been activated during VRC01 therapy, causing treatment failure. Current assays preclude testing this hypothesis experimentally. We developed a mathematical model based on the hypothesis and challenged it with available clinical data. The model integrated within-host HIV-1 evolution, stochastic latency reactivation, and viral dynamics with multiple-dose VRC01 pharmacokinetics. The model predicted that single but not higher VRC01-resistant mutants would pre-exist in the latent reservoir. We constructed a virtual patient population that parsimoniously recapitulated inter-patient variations. Model predictions with this population quantitatively captured data of VRC01 failure from clinical trials, presenting strong evidence supporting the hypothesis. We attributed VRC01 failure to single-mutant VRC01-resistant proviruses in the latent reservoir triggering viral recrudescence, particularly when VRC01 was at trough levels. Pre-existing resistant proviruses in the latent reservoir may similarly compromise other bNAbs. Our study provides a framework for designing bNAb-based therapeutic protocols that would avert such failure and maximize HIV-1 remission.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

