The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: Results from a real-world data analysis
- PMID: 33253294
- PMCID: PMC7703907
- DOI: 10.1371/journal.pone.0242922
The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: Results from a real-world data analysis
Abstract
Background: Although randomized trials provide a high level of evidence regarding the efficacy of non-vitamin K oral anticoagulants (NOACs), the results of such trials may differ from those observed in day-to-day clinical practice.
Aims: To compare the risk of stroke/systemic embolism (S/SE) and major bleeding (MB) between NOAC and warfarin in clinical practice.
Methods: Patients with non-valvular atrial fibrillation (NVAF) who started warfarin/NOACs between January 2015 and November 2016 were retrospectively identified from Korea's nationwide health insurance claims database. Using inpatient diagnosis and imaging records, the Cox models with inverse probability of treatment weighting using propensity scores were used to estimate hazard ratios (HRs) for NOACs relative to warfarin.
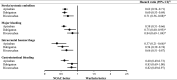
Results: Of the 48,389 patients, 10,548, 11,414, 17,779 and 8,648 were administered apixaban, dabigatran, rivaroxaban and warfarin, respectively. Many patients had suffered prior strokes (36.7%, 37.7%, 31.4%, and 32.2% in apixaban, dabigatran, rivaroxaban, and warfarin group, respectively), exhibited high CHA2DS2-VASc (4.8, 4.6, 4.6, and 4.1 in apixaban, dabigatran, rivaroxaban, and warfarin group, respectively) and HAS-BLED (3.7, 3.6, 3.6, and 3.3 in apixaban, dabigatran, rivaroxaban, and warfarin group, respectively) scores, had received antiplatelet therapy (75.4%, 75.7%, 76.8%, and 70.1% in apixaban, dabigatran, rivaroxaban, and warfarin group, respectively), or were administered reduced doses of NOACs (49.8%, 52.9%, and 42.8% in apixaban, dabigatran, and rivaroxaban group, respectively). Apixaban, dabigatran and rivaroxaban showed a significantly lower S/SE risk [HR, 95% confidence intervals (CI): 0.62, 0.54-0.71; 0.60, 0.53-0.69; and 0.71, 0.56-0.88, respectively] than warfarin. Apixaban and dabigatran (HR, 95% CI: 0.58, 0.51-0.66 and 0.75, 0.60-0.95, respectively), but not rivaroxaban (HR, 95% CI: 0.84, 0.69-1.04), showed a significantly lower MB risk than warfarin.
Conclusions: Among Asian patients who were associated with higher bleeding risk, low adherence, and receiving reduced NOAC dose than that provided in randomised controlled trials, all NOACs were associated with a significantly lower S/SE risk and apixaban and dabigatran with a significantly lower MB risk than warfarin.
Conflict of interest statement
Authors Y-J. Park, J-M. Lee, and S. Kang are full-time employees and stockholders of Pfizer Inc. M-M. Won and H-Y. Choi are previously employees of Pfizer Inc. in connection with the development of this manuscript. Authors O-Y. Bang, Y-K. On, M-Y. Lee, S-W. Jang, S. Han, S. Han, H-S. Suh, and Y-H. Kim are paid consultants for Pfizer Korea Ltd. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

